We report the case of an 89-year-old man who developed composite gastric cancer and Diffuse Large B-Cell Lymphoma (DLBCL) arising from the remnant stomach. He had undergone partial gastrectomy for benign gastric ulcer 60 years earlier. Upper gastrointestinal series from a periodic health examination showed an ulceration in the remnant stomach near anastomotic site, and a biopsy from endoscopic examination revealed gastric cancer. He underwent total gastrectomy, and histological examination revealed composite gastric cancer and DLBCL. Lymphadenopathy was not detected on pre- or post-operative Computed Tomography (CT), and the cancer was considered limited. Helicobacter pylori and Epstein-Barr virus were not detected. Although composite gastric cancer and DLBCL arising from the remnant stomach is considered extremely rare, particular attention should be given to the residual stomach after partial gastrectomy.
Gastric cancer; Diffuse large B-cell lymphoma; Remnant stomach
Gastric cancer remains a serious problem both in Japan and worldwide, although the incidence and mortality have decreased. Most malignant neoplasms of the stomach are gastric cancer, and the incidence of malignant lymphoma of the stomach is estimated to be 5%, with Diffuse Large B-Cell Lymphoma (DLBCL) comprising 59% of those cases [1]. Cancer of the gastric remnant is defined as carcinoma arising in the remnant stomach at ³5 years after partial gastrectomy for benign disease [2,3]. The incidence of gastric stump carcinoma is estimated to be 1-2% [4,5], and few reports have described malignant lymphoma developing from the remnant stomach [6-8]. Similarly, composite development of gastric cancer and malignant lymphoma is extremely rare and only a few cases have been reported. We report herein a case of composite development of gastric cancer and malignant lymphoma 60 years after gastrectomy for benign gastric ulcer.
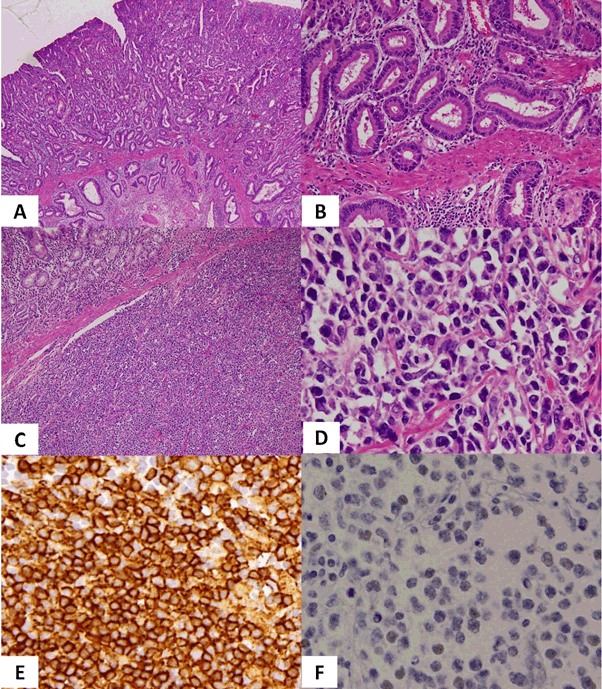
An 89-year-old man was referred to our hospital for an abnormality identified in an upper gastrointestinal series during a periodic health examination. He had undergone partial gastrectomy for benign gastroduodenal ulcer 60 years earlier. Prostate cancer had been diagnosed from prostate biopsy 1 year earlier, and was observed without treatment because the patient was elderly. Tamsulosin hydrochloride was administered for prostatic hyperplasia. Endoscopic examination revealed an ulcerative legion and tumour at the lesser curvature of the remnant stomach near the anastomotic site, and histological examination of a biopsy showed stomach cancer. Preoperative Computed Tomography (CT) scan and abdominal ultrasound examination did not show lymphadenopathy, distant metastasis or ascites. Colonoscopy showed one colon polyp and histological examination revealed no malignancy. Total resection of the remnant stomach and lymph node dissection were performed. The postoperative course was uneventful. Pathological findings for the removed stomach showed well-differentiated adenocarcinoma (Figure 1A, B). As the removed lymph nodes showed no findings suggestive of metastatic gastric cancer, this case of gastric cancer was diagnosed as stage 1A of pT1b2 (SM2), pN0, M0. In addition, diffuse proliferation of medium-sized atypical cells was seen infiltrating from the submucosa layer to the serosa and immunohistochemical staining revealed positive results for CD10, CD20 and CD79a, and negative results for AE1/AE3, CD3, CD5, MUM1, BCL2, BCL6 and Epstein-Barr Virus (EBV)-Encoded small RNA (EBER), resulting in the diagnosis of DLBCL (Figure 1C-F). Helicobacter pylori was not observed. As a result, this case was diagnosed as showing composite gastric cancer and primary gastric DLBCL arising from the remnant stomach. He chose best supportive care after postoperative chemotherapy had been recommended. Postoperative whole-body CT showed neither lymphadenopathy nor distant metastasis, so the DLBCL was diagnosed as limited stage. Blood tests revealed that Lactate Dehydrogenase (LDH) was within the normal range, while soluble interleukin 2 receptor was mildly increased to 641 U/ml (normal range, 145-519 U/ml). Four months after surgery, the patient presented again with anorexia. CT of the whole body showed abdominal lymphadenopathies near the anastomotic site and an upper gastrointestinal series showed strong stenosis ahead of proximal to the anastomotic site. The patient received palliative treatment and died 6 months after surgery.
We encountered a case of composite gastric cancer and DLBCL arising from the remnant stomach. Gastric cancer accounts for the majority of primary gastric malignant tumours and the incidence of malignant lymphoma is low (5%) [1]. The incidence of cancer arising from the remnant stomach has been reported as 1-2% [4,5] and lymphoma arising from the remnant stomach is rare [6-8]. Concurrent onset of gastric cancer and lymphoma thus seems extremely rare [9,10]. In this case, gastric cancer was histologically well-differentiated and DLBCL was the Germinal Center B-cell (GCB) type, which shows a better prognosis than non-GCB type [11]. Both tumours were limited to remnant stomach on CT, and the gastric cancer was completely resected. The patient therefore did not receive additional treatment considering his age, although appropriate postoperative chemotherapy might have offered some survival benefit.
Etiologically H. pylori infection is well known as a major factor in the development of gastric malignancy [5]. Gastric lymphoma in particular appears related to mucosa-associated lymphoid tissue lymphoma [6]. Also, EBV is well known to cause various tumours, including malignant lymphoma and gastric cancer [5]. In this case, negative results were obtained for both H. pylori and EBV. One possibility is that tumorigenesis was triggered by the immunocompromised background with advanced age and prostate cancer. In this case, it was very difficult to diagnose composite tumours before pre-operative biopsy. The biopsy tissues were very small, and DLBCL cells was seen infiltrating from the submucosa layer to the serosa, not in mucosa. No lesions other than gastric cancer were found on pre- or post-operative CT, abdominal ultrasonography, gastroscopy or colonoscopy. Since positron emission tomography/CT was not performed, we could not deny the possibility that the primary site for DLBCL was outside the remnant stomach. We could not examine for genetic abnormalities of DLBCL, such as double-hit lymphoma, which would have suggested a poor prognosis.
We encountered an extremely rare case of gastric cancer and DLBCL arising from the remnant stomach. This pathology is rare, but particular attention should be given to the residual stomach after partial gastrectomy.
The authors have no conflicts of interest to disclose.
- Juárez-Salcedo LM, Sokol L, Chavez JC, Dalia S (2018) Primary gastric lymphoma, epidemiology, clinical diagnosis, and treatment. Cancer Control. 25: 1073274818778256. [Crossref]
- Pointner R, Schwab G, Königsrainer A, Bodner E, Schmid KW (1989) Gatric stump cancer: etiopathological and clinical aspects. Endoscopy. 21: 115-119. [Crossref]
- Thorban S, Böttcher K, Etter M, Roder JD, Busch R, et al. (2000) Prognostic factors in gastric stump carcinoma. Ann Surg. 231: 188-194. [Crossref]
- Lundegårdh G, Adami HO, Helmick C, Zack M, Meirik O (1988) Stomach cancer after partial gastrectomy for benign ulcer disease. N Engl J Med. 319: 195-200. [Crossref]
- Takeno S, Hashimoto T, Maki K, Shibata R, Shiwaku H, et al. (2014) Gastric cancer arising from the remnant stomach after distal gastrectomy: a review. World J Gastroenterol. 20: 13734-13740. [Crossref]
- Greco L, Marino F, Troilo VL, Marzullo A, Gentile A (2006) Gastric stump lymphoma after distal gastrectomy for benign peptic ulcer: Report of a case. Surg Today. 36: 985-988. [Crossref]
- Di Cosimo S, Ferretti G, Partenzi A, Manicone AM, D'Aprile M (2003) Gastric stump lymphoma five years after distal gastrectomy. Leuk Lymphoma. 44: 365-367. [Crossref]
- Oshita H, Tanemura H, Kanno A, Kusakabe M, Hato T, et al. (2003) Malignant lymphoma occurring in the residual stomach following gastrectomy: plus, discussion based on the literature in Japan. Gastric Cancer. 6: 60-63. [Crossref]
- Brouland JP, Manivet P, Brocheriou-Spelle I, Wassef M, Le Bodic MF, et al. (2001) Histological, immunohistochemical, ultrastructural and biochemical study of human gastric composite tumor: expression of the serotonin-2B receptor by the neuroendocrine component. Endocr Pathol. 12: 77-86. [Crossref]
- Kheiri B, Osman M, Congdon D, Bachuwa G (2017) A rare case of gastric mixed adenoneuroendocrine carcinoma (MANEC) with gastric Heliocobacter pylori-negative mucosa-associated lymphoid tissue (MALT) lymphoma. BMJ Case Rep. 14: 2017. [Crossref]
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, et al. (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 103: 275-282. [Crossref]