Lonicera japonica Thunb is one of the most important genera in the Caprifoliaceae family and plays a vital role in traditional Chinese medicine prescriptions. All aerial parts (leaf, flower bud, flower, and caulis) of it can be used as herbal medicines for different traditional efficacy. Plant metabolomics provides a powerful tool for better differentiation and discovery of chemical marker. In the present work, a strategy for integrating metabolic profiling and multi-step PLS-DA analysis was developed to separate the different aerial parts and reveal the chemical markers of L. japonica. The strategy consists of two portions. One is an ultra-fast liquid chromatography coupled with triple quadrupole-time of flight tandem mass spectrometry (UFLC-Triple QTOF MS/MS) method, which was employed to explore chemical compositions. The other is multi-step PLS-DA, which was applied to distinguish the different aerial parts and to reveal the differential characteristic metabolites. A total of 71 metabolites were identified from samples, followed by 8 candidate compounds (Lonicerin, Kaempferol-3-O-rutinoside, Loganin, Isochlorogenic acid B, Isochlorogenic acid C, Secologanic acid, Luteoloside, Astragalin) as optimal chemical markers based on VIP and p-value. This study not only established an efficient strategy for exploring metabolite profiling and finding chemical markers among the different aerial parts of L. japonica, but also laid the foundation for elucidating the differences in efficacy between Lonicerae Japonicae Flos (LJF) and Lonicerae Japonicae Caulis (LJC) at the phytochemistry level. Meanwhile, it also implied from the perspective of structure-activity relationship that leaf could be used as an alternative medicinal resource for LJF.
Lonicera japonica Thunb; UFLC-Triple TOF-MS/MS; metabolic profiling; multivariate statistical analysis
Lonicera japonica Thunb. is native to the countries of eastern Asia [1], it is one of the most important genera in the Caprifoliaceae family and plays a vital role in traditional Chinese medicine prescriptions. Some species and different parts of Lonicera have been historically utilized as herbal medicines against a variety of diseases, including exopathogenic wind-heat, epidemic febrile diseases, sores, swelling, carbuncles, furuncles, erysipelas, and some infectious diseases [2]. At the same time, it is also used to make tea, cosmetics and healthy beverage all over the world.
Lonicerae Japonicae Flos (LJF) and Lonicerae japonicae Caulis (LJC) both come from Lonicera japonica Thunb, but the medicinal parts are different. LJF is derived from the dried buds and flowers [3], while LJC is originated from dried stems [4]. LJF germinates in ovoid leaves, and then blooming white and yellow flowers, which is sweetly scented in the early summer and traditionally harvested at that time. One of the semi-evergreen entangled woody vines, LJC is harvested in autumn and winter. The efficacy of LJF and LJC recorded is different in Chinese Pharmacopoeia. However, the reasons for these differences are still unclear. It is universally acknowledged that chemical composition is the basis of the pharmacological activity of medicinal materials and different chemical compositions have a different bearings on the clinical efficacy. For a long time, it is still a bottleneck in view of the elusive component-effect correlation of Traditional Chinese Medicines (TCMs). There are also many factors that may lead to chemical compositions differences, such as harvest season [5,6], processed methods [7], biological and abiotic factors [8-10], etc. Therefore, the systematic study of chemical composition is necessary. This is also a precondition for identifying chemical markers that differentiate between LJF and LJC, and it may reflect different properties of them.
Metabolomic approach [11,12] focuses on the analysis of holistic metabolites in the biological system, which has been widely applied in natural product characterization, the analysis of biological metabolites with low molecular weight, food quality evaluation and so forth. However, untargeted metabolomics research on analyzing the chemical composition of L. japonica (different parts and different harvesting time of flower) has not been reported to date. In such classic works as ‘Ming Yi Bie Lu’ listed the leaf of L. japonica as a top-grade herb, described: “...like the stems, the winter is not withered”, “December, dried”. The young leaves are also used as spring greens in Korea [13]. And the yield is about 10 times that of the flower [14]. Nevertheless, the leaf is usually discarded because it is a non-medicinal part of L. japonica, resulting in underutilization and a great loss of potential resources. With the increasing demand for LJF, the leaf has attracted considerable attention. Some pharmacological studies also have demonstrated that the leaf possesses many biological functions, such as antibacterial [15], anti-AIV [16], antioxidant [17], and hepatoprotective effects.
In this work, we developed a strategy integrating metabolic profiling and multi-step PLS-DA analysis to separate the different aerial parts (including Leaf, Flower Bud, Flower, and Caulis) and discover chemical markers of them. Hence, an effective method was proposed to profile the primary and secondary metabolites in leaf, flower bud, flower, and caulis via ultra-fast liquid chromatography coupled with triple quadrupole-time of flight tandem mass spectrometry (UFLC-Triple TOF-MS/MS). Then, based on these data of metabolite profiling, multi-step PLS-DA were applied to discriminate these samples, and further to reveal the differential compositions among them. The untargeted metabolomics study demonstrated that the chemical composition in flower bud, flower, caulis and leaf of L. japonica had marked difference. A total of 71 metabolites were identified from L. japonica samples. Then, in accordance with a series of comparisons and permutations, Lonicerin, Kaempferol-3-O-rutinoside, Loganin, Isochlorogenic acid B, Isochlorogenic acid C, Secologanic acid, Luteoloside, Astragalin could be selected as optimal chemical markers based on VIP and p-value to distinguish the aerial parts in practice. Last but not least, the leaf exhibited the most similar chemical composition with flower bud and flower, which implied that the leaf could be used as an alternative medicine resource for LJF. Our work is not only beneficial to reduce the waste of resources, but also provides a reference for comprehensive exploitation and utilization of L. japonica resources.
Identification of chemical constituents
In our study, chemical compounds of samples were analyzed and identified using ESI-MS/MS, and the best analytical selectivity and sensitivity were obtained in the negative ionization mode. The total chromatograms were presented in figure 1.
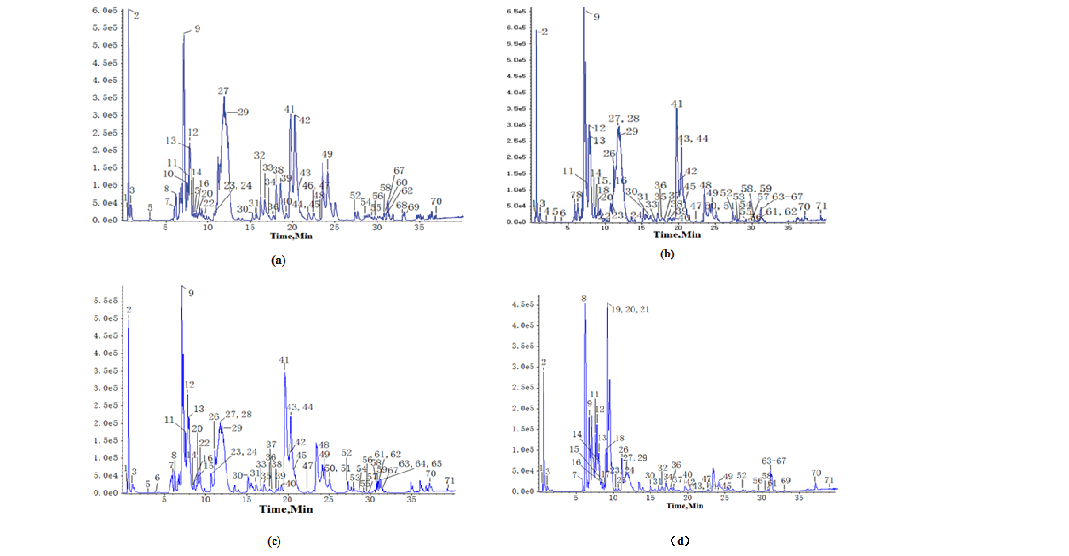
Figure 1. Representative UPLC/ESI-QTOF-MS BPI (Base peak intensity) profiles of samples ((a) Leaf (b) Flower bud (c) Flower (d) Caulis) in the negative
According to the database that we built, reference standards, and related literature, 71 metabolites were identified, including 4 alkaloids, 24 organic acids, 15 iridoids, 23 flavonoids, and 5 saponins. Most of the compounds were identified through comparation with the retention time and characteristic fragment ions of standard substances; some compounds were obtained based on the data that reported in relative literature. The details of identified compounds from leaf, flower bud, flower, and caulis were summarized in Table 1; the structures of these compounds were provided in figure 2.
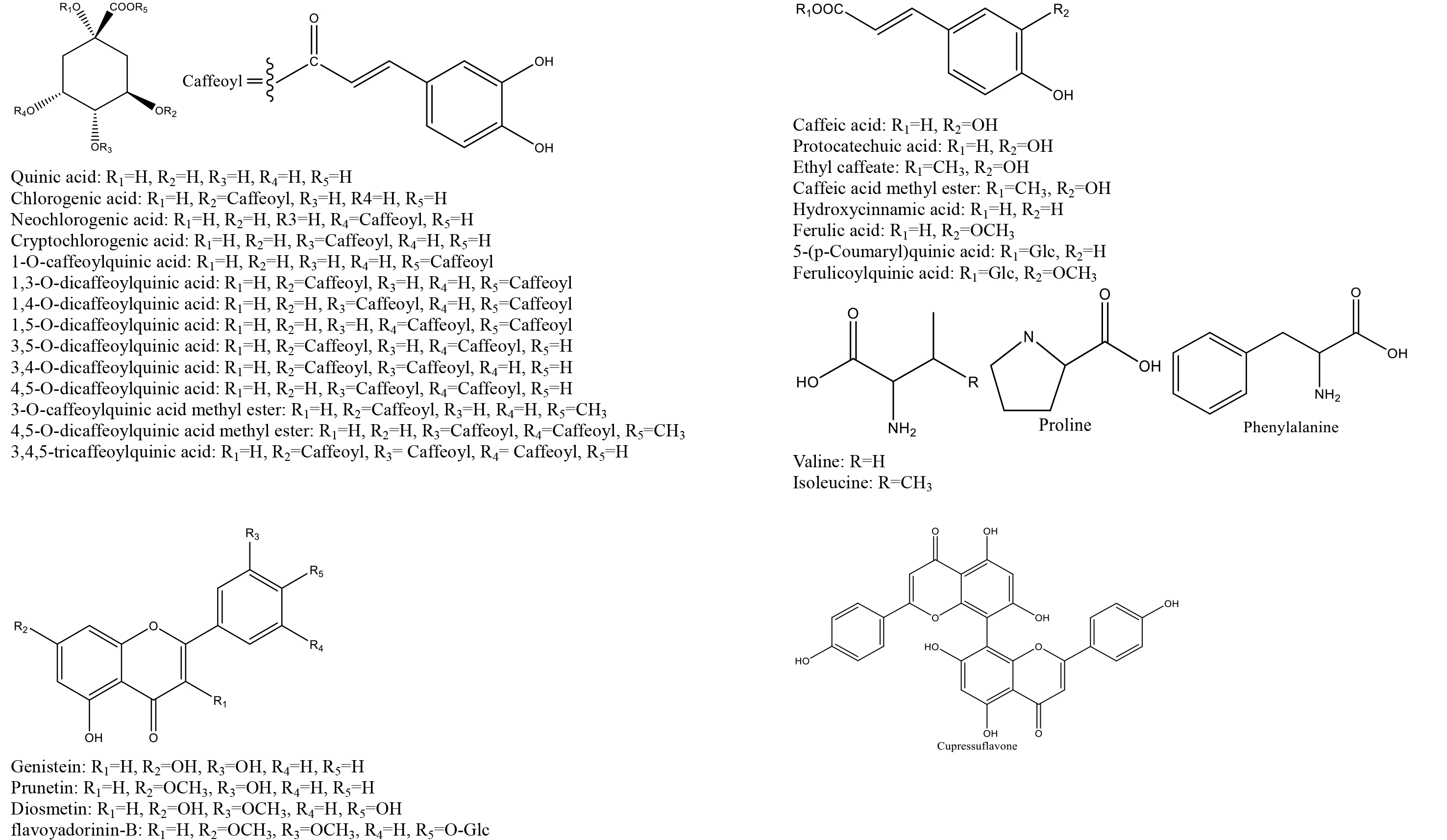
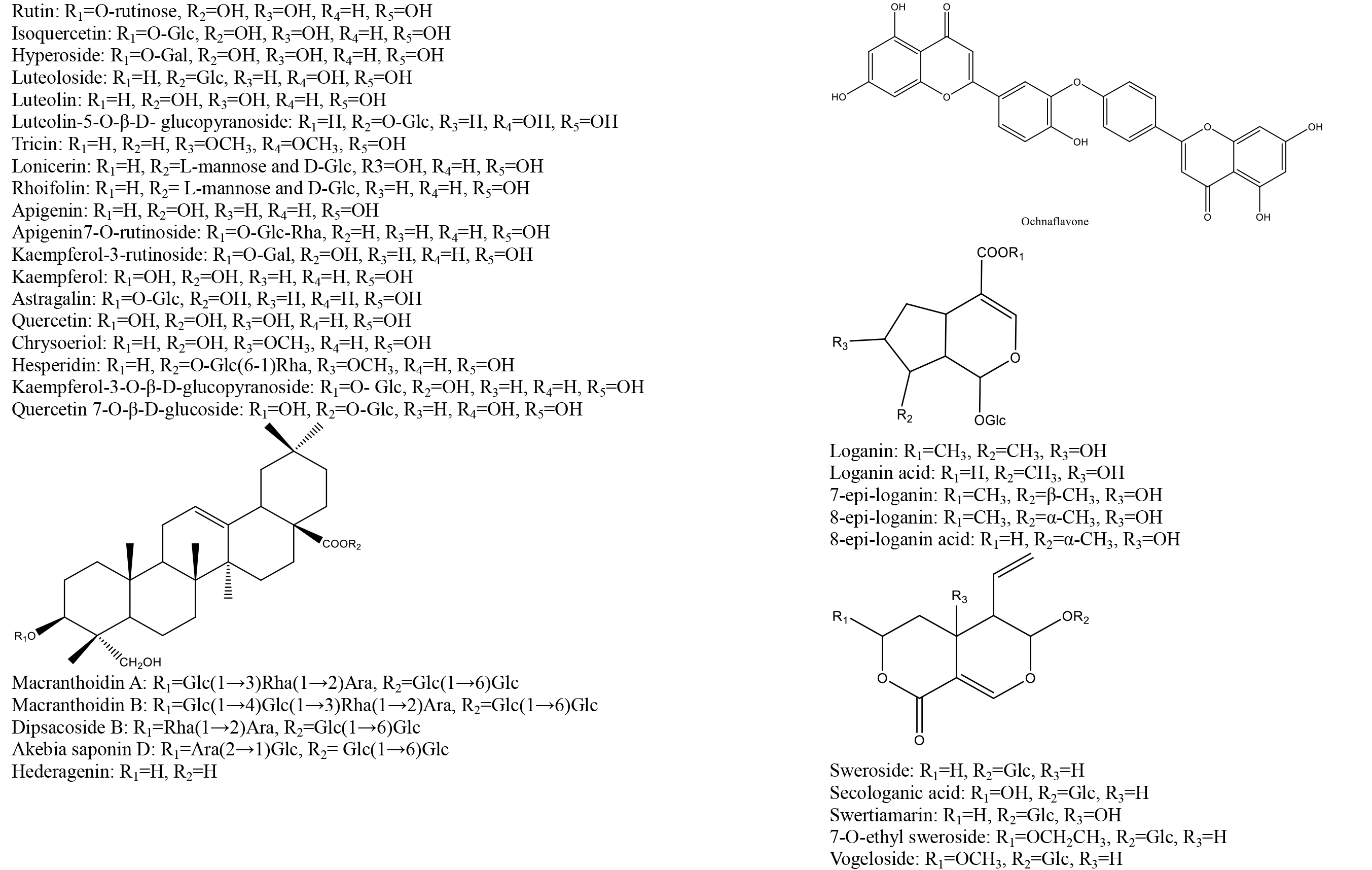
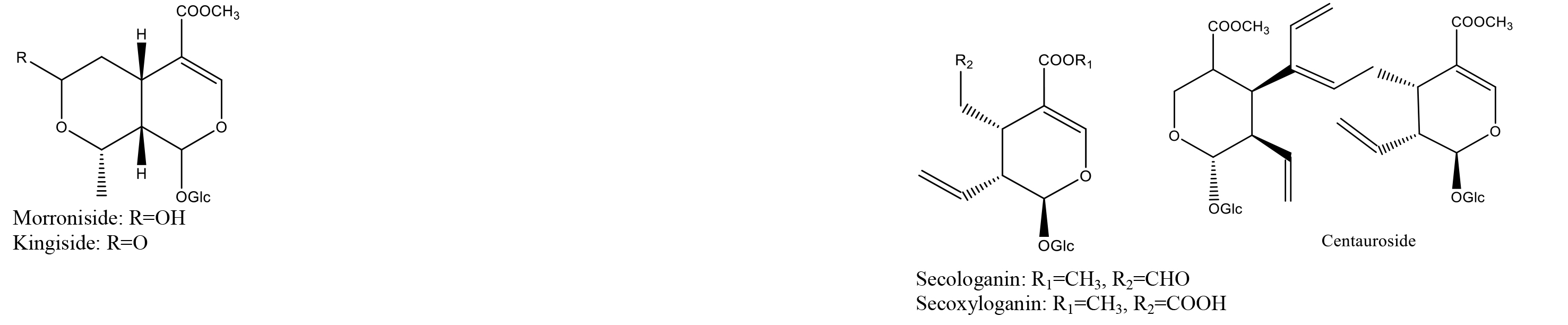
Figure 2. Chemical structures of identified compounds from aerial parts of Lonicera japonica Thunb.
Table 1. Identification of 71 metabolites in the aerial parts of Lonicera japonica Thunb. by UFLC-Triple TOF-MS/MS
No. |
RT(min) |
Molecular formula |
Proposal ions |
ppm |
MS/MS fragment |
Identification |
Classification |
Aerial parts |
Reference |
Leaf |
Flower bud |
Flower |
Caulis |
1 |
0.49 |
C5H9NO2 |
[M-H]- |
4.5 |
114.9905[M-H]-,56.9982[M-H-C3H3O2-CH3]- |
Proline* |
alkaloid |
+ |
+ |
+ |
+ |
|
2 |
0.61 |
C7H12O6 |
[M-H]- |
0.2 |
191.05615-,173,154 |
Quinic acid* |
organic acid |
+ |
+ |
+ |
+ |
22 |
3 |
0.95 |
C5H11NO2 |
[M-H]- |
5.4 |
101.0608[M-H-NH3]-,85.0295[M-H-NH3-CH4]-,57.0710[M-H-C2H4O2]- |
Valine* |
alkaloid |
+ |
+ |
+ |
+ |
|
4 |
2.09 |
C6H13NO2 |
[M-H]- |
3.8 |
130.874[M-H]-,98.0611[M-H2O-CH3]- |
Isoleucine* |
alkaloid |
- |
+ |
- |
- |
|
5 |
3.32 |
C9H11NO2 |
[M-H]- |
2.8 |
146.0611[M-H-H2O]-,103.0553[M-H-COO-NH3]-, 77.0397[M-H-COO-NH3-C2H2]- |
Phenylalanine* |
alkaloid |
- |
+ |
+ |
- |
23 |
6 |
4.1 |
C7H6O4 |
[M-H]- |
2.4 |
109.0295[M-H-COO]-, 91.0189[M-H-COO-H2O]- |
Protocatechuic acid* |
organic acid |
- |
+ |
+ |
- |
24 |
7 |
6.09 |
C16H24O10 |
[M-H]- |
3.5 |
213.0759[M-H-Glc]-,167.0447[M-H-Glc-H2O-CO]- |
8-epi-loganin acid |
iridoid |
- |
+ |
+ |
+ |
22,25,29 |
8 |
6.41 |
C16H18O9 |
[M-H]- |
3.1 |
191.0569[M-H-CA]-,179.0351,135.0456 |
1-O-caffeoylquinic acid* |
organic acid |
+ |
+ |
+ |
+ |
|
9 |
7.5 |
C16H18O9 |
[M-H]- |
-1.1 |
191.0568[M-H-CA]-,127.0398[[M-H-CA-2H2O-CO]- |
Chlorogenic acid* |
organic acid |
+ |
+ |
+ |
+ |
26,27 |
10 |
7.49 |
C17H26O11 |
[M+HCOO]- |
4.1 |
451.1476[M-H+HCOOH]-,243.0899[M-H-Glc]-,191.0573,119.0368,105.0323,101.0263 |
Morroniside* |
iridoid |
+ |
- |
- |
- |
|
11 |
7.1 |
C16H24O10 |
[M-H]- |
3.8 |
329.1492[M-H-H2O-CO]-,213.0759[M-H-Glc]-,169.0871[M-H-Glc-CO2]-,151.0765[M-H-Glc-CO2-H2O]- |
Loganic acid* |
iridoid |
+ |
+ |
+ |
+ |
22,29 |
12 |
7.79 |
C16H18O9 |
[M-H]- |
3.1 |
191.0569[M-H-CA]-,179.0351,135.0456 |
Neochlorogenic acid* |
organic acid |
+ |
+ |
+ |
+ |
26,27 |
13 |
7.9 |
C16H18O9 |
[M-H]- |
-1.1 |
191.0568[M-H-CA]-,127.0398[M-H-CA-2H2O-CO]- |
Cryptochlorogenic acid* |
organic acid |
+ |
+ |
+ |
+ |
26,27 |
14 |
8.1 |
C9H8O4 |
[M-H]- |
2.9 |
135.0460[M-H-CO2]- |
Caffeic acid* |
organic acid |
+ |
+ |
+ |
+ |
27 |
15 |
8.37 |
C16H22O10 |
[M-H]- |
2.8 |
193.0523[M-H-Glc-H2O]-,149.0617[M-H-Glc-H2O-CO2]-, 119.0356,101.0250 |
Swertiamarin |
iridoid |
+ |
+ |
+ |
+ |
23 |
16 |
8.37 |
C16H22O10 |
[M-H]- |
2.9 |
193.0523[M-H-Glc-H2O]-,149.0617[M-H-Glc-CO2-H2O]-, 141.0189,123.0464,119.0356,105.0344,101.0250 |
Secologanic acid* |
iridoid |
+ |
+ |
+ |
+ |
22,29 |
17 |
8.95 |
C11H12O4 |
[M+HCOO]- |
2.1 |
161.0329[M-H-C2H5O]-,135.0468,133.0282[M-H-C2H5O-CO]- |
Ethyl caffeate |
organic acid |
- |
- |
- |
+ |
27 |
18 |
9.03 |
C17H24O10 |
[M-H]- |
-3.6 |
341.1093[M-H-H2O-CO]-,179.0544[M-H-Glc-H2O-CO]-, 161.0444,149.0440,131.0336,119.0354,101.0247 |
Secologanin* |
iridoid |
+ |
+ |
- |
- |
|
19 |
9.2 |
C17H26O10 |
[M+HCOO]- |
3.4 |
227.0932[M-H-Glc]-,209.0987[M-H-Glc-H2O]-,191.0563[M-H-Glc-2H2O]-,153.0697,149.0785,129.0559 |
7-epi-loganin |
iridoid |
- |
- |
- |
+ |
25,29 |
20 |
9.23 |
C18H26O10 |
[M-H]- |
2.8 |
175.0412[M-H-Glc-CH3CH2OH-H2O]- |
7-O-ethyl sweroside |
iridoid |
+ |
+ |
+ |
+ |
28 |
21 |
9.23 |
C17H26O10 |
[M+HCOO]- |
4.6 |
227.0911[M-H-Glc]-,209.0809[M-H-Glc-H2O]-,191.0562[M-H-Glc-2H2O]-,133.0303,101.0250 |
8-epi-loganin |
iridoid |
- |
- |
- |
+ |
|
22 |
9.32 |
C16H18O8 |
[M-H]- |
3.2 |
191.0575[QA-H]-,173.0442[QA-H-H2O]-,163.0391[M-H-QA]-,127.0387[QA-H-2H2O-CO]-, 117.0323[PA-H2O-CO]- |
5-(p-Coumaryl) quinic acid |
organic acid |
+ |
+ |
+ |
+ |
23 |
23 |
10.78 |
C17H20O9 |
[M-H]- |
4.7 |
191.0571[QA-H]-,173.0469[M-H-CA-CH3OH]-,127.0401[M-H-CA-CH3OH-H2O-CO]- |
3-O-caffeoylquinic acid methyl ester |
organic acid |
+ |
+ |
+ |
+ |
23 |
24 |
10.78 |
C17H20O9 |
[M-H]- |
4.7 |
191.0571[QA-H]-,173.0469[QA-H-H2O]-,127.0401[QA-H-2H2O-CO]-,117.0360[M-H-QA-OCH3-CO]- |
3-O-ferulicoylquinic acid |
organic acid |
+ |
+ |
+ |
+ |
23 |
25 |
11.42 |
C10H10O4 |
[M-H]- |
0.15 |
149.0608[M-H-COO]-,133.0295[M-H-COOH-CH3]- |
Ferulic acid* |
organic acid |
- |
- |
- |
+ |
|
26 |
11.47 |
C17H26O10 |
[M+HCOO]- |
6.1 |
389.1781[M-H]-,227.1144[M-H-Glc]- |
Loganin* |
iridoid |
- |
+ |
+ |
+ |
26,29 |
27 |
11.8 |
C17H24O10 |
[M+HCOO]- |
4.7 |
175.0402[M-H-Glc-CH3OH-H2O]-,151.0358[M-H-Glc-C4H6O]-,149.0591[M-H-Glc-CH3OH-H2O-C2H2]-, 119.0347,101.0251 |
Vogeloside |
iridoid |
+ |
+ |
+ |
+ |
22, 26,29 |
28 |
11.88 |
C17H24O11 |
[M-H]- |
3.9 |
165.0569,149.0262[M-H-Glc-CH3-COO-H2O-CH3]-, 121.0311,119.0382,101.0257 |
Kingiside |
iridoid |
- |
+ |
+ |
- |
22 |
29 |
11.9 |
C17H24O11 |
[M-H]- |
3.9 |
149.0252[M-H-Glc-H2O-CH3OH-C2H2O]-,121.0308[M-H-Glc-H2O-CH3OH-C2H2O-CO]- |
Secoxyloganin* |
iridoid |
+ |
+ |
+ |
+ |
22,29 |
30 |
15.18 |
C21H20O12 |
[M-H]- |
3.4 |
301.0387[M-H-Glc]-,283.0131[M-H-Glc-H2O]-, 151.0054,107.0174 |
Hyperoside* |
flavonoid |
+ |
+ |
+ |
+ |
22,29 |
31 |
16.01 |
C27H30O16 |
[M-H]- |
-1.8 |
301.0374[M-H-Rha-Glc]- |
Rutin* |
flavonoid |
+ |
+ |
+ |
+ |
29 |
32 |
16.56 |
C21H20O12 |
[M-H]- |
5.8 |
301.0374[M-H-Glc]-,271.0265[M-H-Glc-CH2O]-,151.0033 |
Quercetin-7-O-glucoside |
flavonoid |
+ |
- |
- |
+ |
|
33 |
16.68 |
C21H20O12 |
[M-H]- |
3.9 |
301.0379[M-H-Glc]-,151.0044 |
Isoquercitrin* |
flavonoid |
+ |
+ |
+ |
- |
|
34 |
17.08 |
C21H20O11 |
[M-H]- |
2.2 |
285.0418[M-H-Glc]- |
Luteolin-5-O-β-D- glucopyranoside |
flavonoid |
+ |
- |
- |
+ |
|
35 |
17.17 |
C21H20O11 |
[M-H]- |
3.3 |
285.0419[M-H-Glc]- |
Luteoloside* |
flavonoid |
- |
+ |
+ |
- |
|
36 |
17.98 |
C27H30O15 |
[M-H]- |
0.01 |
285.0405[M-H-Glc-Rha]- |
Lonicerin* |
flavonoid |
+ |
+ |
+ |
+ |
22,29 |
37 |
18.04 |
C28H34O15 |
[M-H]- |
-2.8 |
301.036[M-H-Rha-Glc]-, 271.0265,255.0304,179.0090,151.0033 |
Hesperidin* |
flavonoid |
- |
+ |
+ |
- |
|
38 |
18.54 |
C25H24O12 |
[M-H]- |
0.6 |
353.0821[M-H-CA]-,335.0821[M-H-CA-H2O]-,191.0567[M-H-2CA]-,179.0377[CA-H]-,173.0481[M-H-2CA-H2O]-,161.0268[CA-H-H2O]-,135.0613[CA-H-CO2]- |
1,3-O-dicaffeoylquinic acid* |
organic acid |
+ |
+ |
+ |
- |
22 |
39 |
19.07 |
C27H30O15 |
[M-H]- |
4.4 |
593.1570,285.0419[M-H-Rha-Glc]- |
Kaempferol-3-O-rutinoside* |
flavonoid |
+ |
+ |
+ |
- |
29 |
40 |
19.49 |
C10H10O4 |
[M-H]- |
4.4 |
133.0305[M-H-CH3-COOH]- |
Caffeic acid methyl ester |
organic acid |
+ |
- |
+ |
+ |
33 |
41 |
20.06 |
C25H24O12 |
[M-H]- |
-2.8 |
353.0924[M-H-CA]-,335.0793[M-H-CA-H2O]-,191.0559[M-H-2CA]-,179.0355,173.0458[M-H-2CA-H2O]-,161.0241[CA-H-H2O]-,155.0346[M-H-2CA-2H2O]-, 135.0459[CA-H-CO]- |
Isochlorogenic acid B* |
organic acid |
+ |
+ |
+ |
- |
22, 26, |
42 |
20.24 |
C25H24O12 |
[M-H]- |
5.6 |
353.0910[M-H-CA]-,335.0816[M-H-CA-H2O]-,191.0575[M-H-2CA]-,179.0369[CA-H]-,173.0472[M-H-2CA-H2O]-, 161.0257[CA-H-H2O]-,135.0464[CA-H-CO2]- |
1,5-O-dicaffeoylquinic acid |
organic acid |
+ |
+ |
+ |
+ |
23 |
43 |
20.6 |
C21H20O11 |
[M-H]- |
2.2 |
285.0428[M-H-Glc]- |
Astragalin* |
flavonoid |
+ |
+ |
+ |
+ |
|
44 |
20.62 |
C25H24O12 |
[M-H]- |
-3.1 |
353.0901[M-H-CA]-,191.0569[M-H-2CA]-,179.0353[CA-H]-, 173.0463[M-H-2CA-H2O]-,135.0457[CA-H-CO]- |
Isochlorogenic acid A* |
organic acid |
+ |
+ |
+ |
- |
22, 26, |
45 |
20.67 |
C25H24O12 |
[M-H]- |
6.2 |
353.0905[M-H-CA]-,191.0576[M-H-2CA]-,179.0366[CA-H]-, 173.0460[M-H-2CA-H2O]-,161.0247[CA-H-H2O]-, 135.0460[CA-H-CO]- |
1,4-O-dicaffeoylquinic acid |
organic acid |
+ |
+ |
+ |
+ |
22 |
46 |
22.61 |
C27H30O14 |
[M-H]- |
-1.3 |
269.0490[M-H-Rha-Glc]-,191.0582 |
Apigenin-7-O-rutinoside |
flavonoid |
+ |
- |
- |
- |
23,27 |
47 |
22.68 |
C27H30O14 |
[M-H]- |
4.5 |
413.0799[M-H-Rha-H2O]-,269.0462[M-H-Rha-Glc]- |
Rhoifolin* |
flavonoid |
+ |
+ |
+ |
+ |
30 |
48 |
23.09 |
C25H24O12 |
[M-H]- |
-3.1 |
353.0904[M-H-CA]-,191.0575[M-H-2CA]-,179.0360[CA-H]-, 173.0463[M-H-2CA-H2O]-,155.0364[M-H-2CA-2H2O]-, 135.0464[CA-H-CO]- |
Isochlorogenic acid C* |
organic acid |
+ |
+ |
+ |
- |
22 |
49 |
24.52 |
C34H46O19 |
[M-H]- |
0.3 |
725.2528[M-H-OCH3]-,595.1979[M-H-Glc]-,525.1657[M-H-Glc-CH2-C2O2]-,179.0565 |
Centauroside |
iridoid |
+ |
+ |
+ |
+ |
28 |
50 |
24.81 |
C25H24O11 |
[M-H]- |
4.8 |
353.0935[M-H-PA]-,191.0560[QA-H]-,179.0347[CA-H]-, 173.0431[QA-H-H2O]-,163.0410[PA-H]-,161.0258[CA-H-H2O]-,135.0444[CA-H-H2O-CO]-,119.0476 |
Coumaroyl caffeoylquinic acid |
organic acid |
+ |
+ |
+ |
- |
23,25 |
51 |
24.82 |
C25H24O11 |
[M-H]- |
5.4 |
353.0879[M-H-PA]-,319.0844[M-H-CA]-,191.0562[QA-H]-, 179.0353[CA-H]-,173.0427[QA-H-H2O]-,163.0407[PA-H]-, 161.0242[CA-H-H2O]-,135.0444[CA-H-H2O-CO]-, 127.0399[PA-H-2H2O]-,119.0476 |
Coumaroyl caffeoylquinic acid isomer |
organic acid |
+ |
+ |
+ |
- |
|
52 |
27.56 |
C23H24O11 |
[M-H]- |
5.2 |
313.0715[M-H-Glc]-,283.0531,279.0164,269.0429,255.030 |
Flavoyadorinin-B |
flavonoid |
- |
+ |
+ |
+ |
|
53 |
28.03 |
C26H26O12 |
[M-H]- |
5.3 |
353.0898[M-H-C10H8O3]-,191.0568[M-H-C10H8O3-CA]-, 179.0366[CA-H]-,173.0460[M-H-C10H8O3-CA-H2O]-, 161.0247[CA-H-H2O]-,155.0379[M-H-C10H8O3-CA-2H2O]-, 135.0460[CA-H-CO]- |
Feruloyl caffeoylquinic acid |
organic acid |
- |
+ |
+ |
- |
23 |
54 |
28.42 |
C15H10O7 |
[M-H]- |
0.2 |
301.0367[M-H]-,193[M-H-ringB]-,151,121,107 |
Quercetin* |
flavonoid |
- |
+ |
+ |
- |
27,32 |
55 |
29.4 |
C26H26O12 |
[M-H]- |
5 |
367.1076[M-H-CA]-,349.0978[M-H-CA-H2O]-, 179.03340[CA-H]-,161.0243[CA-H-H2O]-,135.0447[CA-H-CO2]- |
4,5-O-dicaffeoylquinic acid methyl ester* |
organic acid |
+ |
+ |
+ |
- |
|
56 |
29.66 |
C15H10O6 |
[M-H]- |
4.5 |
199.0399[M-H-H2O-C4H4O]-, 175.0405,151.0026,133.0300,121.0299,107.0151 |
Luteolin* |
flavonoid |
+ |
+ |
+ |
+ |
22,27,29 |
57 |
29.71 |
C15H10O6 |
[M-H]- |
-1.7 |
285.0401,215.0382[M-H-H2O-C4H4]-, 175.0425,151.0027,133.0295 |
Kaempferol* |
flavonoid |
- |
+ |
+ |
- |
32 |
58 |
30.75 |
C34H30O15 |
[M-H]- |
6.3 |
515.1256[M-H-CA]-,353.0907[M-H-2CA]-,335.0794[M-H-2CA-H2O]-,191.0578[QA-H]-,179.0366[CA-H]-,173.0470[QA-H-H2O]-,161.0261[CA-H-H2O]-,135.0454[CA-H-H2O-C2H2]- |
3,4,5-tricaffeoylquinic acid |
organic acid |
+ |
+ |
+ |
- |
22 |
59 |
30.81 |
C59H96O27 |
[M-H]- |
2.5 |
911.5010[M-H-2Glc]- |
Macranthoidin A* |
saponin |
- |
- |
- |
+ |
29 |
60 |
30.85 |
C65H106O32 |
[M+HCOO]- |
-3.5 |
1235.6066[M-H-Glc]-,911.5010[M-H-3Glc]- |
Macranthoidin B |
saponin |
+ |
- |
- |
- |
29 |
61 |
30.91 |
C53H86O22 |
[M+HCOO]- |
1 |
1119.548[M-H+HCOOH]-,749.4482[M-H-2Glc]-,323.0984 |
Dipsacoside B* |
saponin |
- |
+ |
+ |
+ |
29 |
62 |
30.9 |
C15H10O5 |
[M-H]- |
3.4 |
117.0357 |
Apigenin* |
flavonoid |
+ |
+ |
+ |
- |
27,32 |
63 |
31.06 |
C15H10O5 |
[M-H]- |
3.4 |
201.0572[M-H-C3O2]-,159.0517[M-H-C3O2-C2H2O]-,151.0089,107.0107 |
Genistein |
flavonoid |
- |
+ |
+ |
+ |
31 |
64 |
31.09 |
C17H14O7 |
[M-H]- |
-0.1 |
329.6663[M-H]-,211 |
Tricin |
flavonoid |
- |
+ |
+ |
+ |
22 |
65 |
31.2 |
C16H12O6 |
[M-H]- |
-2.6 |
283.0425,255.0315[M-H-C2H40]-, 227.0347,199.0434,151.0044,147.0041,133.0309,107.014 |
Diosmetin* |
flavonoid |
- |
+ |
+ |
+ |
|
66 |
31.2 |
C16H12O6 |
[M-H]- |
6.3 |
283.0425,255.0315[M-H-C2H40]-, 227.0347,199.0434,151.0044,147.0041,133.0309,107.014 |
Chrysoeriol |
flavonoid |
- |
+ |
+ |
+ |
|
67 |
31.22 |
C16H22O9 |
[M+HCOO]- |
4.9 |
151.0809,149.0254[M-H-Glc-H2O-CO]-,125.0263[M-H-Glc-C4H6O]-,119.0062 |
Sweroside* |
iridoid |
+ |
+ |
+ |
+ |
22,28,29 |
68 |
33.06 |
C16H12O5 |
[M-H]- |
5 |
268.0367[M-H-CH3]-,239.0354[M-H-CH3-CHO]-, 171.0526[M-H-CH3-CHO-C3O2]- |
Prunetin |
flavonoid |
+ |
- |
- |
- |
31 |
69 |
33.29 |
C30H18O10 |
[M-H]- |
6.4 |
519.0860[M-H-H2O]-,493.0825[M-H-H2O-C2H2]-, 469.0991[M-H-C4H4O]-,427.0991[M-H-C6H6O2]-, 269.0400[M-H-C15H9O5]-,130.9961 |
Cupressuflavone/Ochanaflavone |
flavonoid |
+ |
- |
- |
+ |
|
70 |
37.32 |
C30H48O4 |
[M+HCOO]- |
6.8 |
517.3570[M-H+HCOOH]-,471.3540,366.9864[M-H-C5H13O2]- |
Hederagenin |
saponin |
+ |
+ |
+ |
+ |
23,29 |
71 |
39.22 |
C47H76O18 |
[M+HCOO]- |
0.01 |
973.4995[M-H+HCOOH]-,927.4959[M-H]-,603.3902[M-H-2Glc]-,323.0984 |
Akebia saponin D* |
saponin |
- |
+ |
+ |
+ |
23 |
Note: * Compare with reference; - Not detected; + detected; QA: quinic acid; CA: caffeic acid; PA: p-coumaric acid
Identification of phenolic acids
In the negative mode, adducted ion of phenolic acid was observed as [M-H]- or [M+HCOO]-. Generally, the basic structure of phenolic acids consists of one or more caffeic acid substituents bound to a portion of quinic acid. Their MS/MS spectra usually owned a basic peak at [M-H-CA]-, and then lost H2O, CO2 or CO, which usually produced various ions for example 353 [M-H-CA]-, 335 [M-H-CA-H2O]-, 179, 135, 127, and so forth. Such as 1-O-caffeoylquinic acid, chlorogenic acid, neochlorogenic acid, and cryptochlorogenic acid showed a characteristic fragment ions at m/z 191, 179,135, 127, dicaffeoylquinic acids and isomers (compound 38, 41, 42, 44, 45, 48) had the same molecular ions at m/z 515 and the secondary fragmentation at 353, 191, 179, 173, 161, 135. The MS/MS fragmentation of compound 3,4,5-tricaffeoylquinic acid lost three caffeoyl moieties at m/z 515, 353, 191, which were consistent with literature reports [18].
Among these phenolic acid compounds, compound 2,6, 8, 9, 12, 13, 14, 25, 38, 41, 44, 48, 55 were identified by comparison on retention time, authentic standards and references. And other compounds were further identified by comparing fragment ions and fragmentation behaviors, which were reported in the relevant literature.
Identification of flavonoids
The fragment rules of flavonoids mainly include the following parts in the negative mode. The first is the flavonoids with a structure of 5,7-OH, which lost C3O2. Such as Genistein produced the fragment ion C3O2 at m/z 201. The second is the different fragmentation patterns of is flavones, flavonoids, and flavonols on the C ring. The fragment ion of m/z 283 [M-H]-, 268 [M-H-CH3]-, 239 [M-H-CH3-CHO]-, 171 [M-H-CH3-CHO-C3O2]- were obtained in the MS/MS, which were coincident with the reference. Thus, compound 68 was acknowledged as prunetin. The third is the RDA reaction pathway of flavonoids and isoflavones on aglycone C ring and the losing the fragment of C2H2O. Such as luteolin, apigenin often occurred RDA reaction pathway. The characteristic fragment ion of the RDA reaction pathway at m/z 151, 133, so compound luteolin was confirmed. In addition, hyperoside, lonicerin, astragalin, flavoyadorinin-B, and luteoloside were loss of glucose, respectively. Rhoifolin, rutin successive lost glucose and rhamnose moieties.
Among these flavonoid compounds, compound 30, 31, 33, 35, 36, 37, 39, 43, 47, 54, 56, 57, 62, 65 were identified by comparison on retention time, authentic standards and references. And other compounds were further identified by comparing fragment ions and fragmentation behaviors, which were reported in the relevant literature.
Identification of iridoid
Most of the iridoid present a relatively high abundant deprotonate molecule [M-H]- in the negative mode. Therefore, the basic peak of iridoid was [M-H]-, and the adduct was [M+HCOO]-. Furthermore, the characteristic fragment ions [M-H-162]- of this category of compounds were formed due to the loss of glucose, and then subsequent loss of H2O, CO or CO2. A total of 15 iridoids were identified from these samples with the TOF-MS/MS data and reference standards. The spectrum of compound 11 showed a fragment ion at m/z 329 [M-H-H2O-CO]-, 213 [M-H-Glc]-,169 [M-H-Glc-CO2]-, 151 [M-H-Glc-CO2-H2O]-, and it was identified as loganic acid. And compound 7-epi-loganin, 8-epi-loganin showed same [M+HCOO]- with loganin at m/z 435. Compound 16 gave several fragment ions at m/z 193 [M-H-Glc-H2O]-, 149 [M-H-Glc-CO2-H2O]-, 141, 123, 119, 105, 101 in the MS/MS spectrum, and it was characterized as secologanic acid. Compound 18 displayed a highly abundant [M-H]-, and its molecular formula was determined to be C17H24O10, it loss of H2O and CO at m/z 341, then loss of glucose generated [M-H-Glc-H2O-CO]- at m/z 179, and produced several fragment ions at m/z 161, 149, 131, 119, 101. Compared with the standard, it was further determined as secologanin.
Among these iridoid compounds, compound 10, 11, 16, 18, 26, 29, 67 were identified by comparison on retention time, authentic standards and references. And other compounds were further identified by comparing fragment ions and fragmentation behaviors, which were reported in the relevant literature.
Identification of saponins
The basic parent nuclei of saponins consisted of aglycone and sugar. In this study, saponins responded more sensitively and had much higher abundance in negative mode. Because the mobile phase contains formic acid, ion of [M-H+HCOOH]- was appeared in most of the saponins. A typical adduct peak was m/z 1119 [M-H+HCOOH]- in the negative mode with 1 ppm error, and then successive loss of glucose at m/z 749 [M-H-2Glc]-. In comparison with the reference standard, compound 61 was assigned as Dipsacoside B.
Among these 5 saponin compounds, compound 59, 61, 71 were identified by comparison on retention time and with authentic standards. And the other compounds were further identified by comparing fragment ions and fragmentation behaviors, which were reported in the relevant literature.
Multivariate statistical analysis
It is the key issue to find the potential chemical marker for distinguishing them. The PCA, multi-step PLS-DA analysis and VIP tests were performed. It could be regarded as a potential marker when the VIP-value of compounds was more than 1.
As shown in figure 3, only LJC was completely separated from the other three parts. The leaf was mingled with flower bud and the flower also mingled in the flower bud. It meant the four different parts were not entirely separated. Hence, PLS-DA was carried out to further identify metabolites that could accurately distinguish the four parts better. (Figure 4). The identification of differential metabolites between every two samples was performed using VIP values. The leaf and caulis were clearly divided into two groups in figure 4(a). Then, the same PLS-DA analysis was conducted for discriminating leaf and LJF (figure. 4(b)), caulis and LJF (figure 4(c)), flower and flower bud (figure 4(d)). They could be completely separated between every two of them. Through comprehensive analysis and comparison, compound 36, 39, 26, 41, 48, 16, 35, 43 (Lonicerin, Kaempferol-3-O-rutinoside, Loganin, Isochlorogenic acid B, Isochlorogenic acid C, Secologanic acid, Luteoloside, Astragalin) had greater contributions to the differentiation of the four parts, which could be considered as the potential compounds to distinguish the four different parts. Finally, compound 36, 39, 26, 41, 48, 16, 35, 43 were set as variables for a new round of PLS-DA analysis of four different parts. As shown in figure. 5 the four different parts were completely discriminated. Therefore, these compounds (Lonicerin, Kaempferol-3-O-rutinoside, Loganin, Isochlorogenic acid B, Isochlorogenic acid C, Secologanic acid, Luteoloside, Astragalin) were selected as optimal markers.
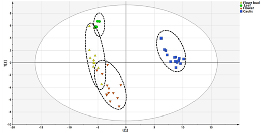
Figure 3. Principal Component Analysis (PCA) scores plot of 44 batches of samples (Green circle represent Leaf samples, Yellow triangles represent Flower bud samples, Orange inverted triangle represent Flower samples, Blue box represent Caulis samples)
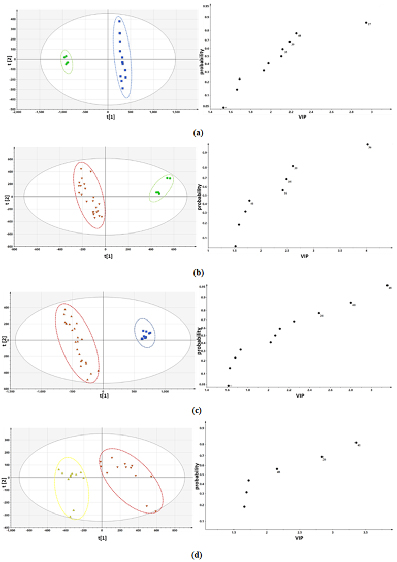
Figure 4. Partial least squares discriminant analysis (PLS‐DA) scores plot and VIP plot of leaf and caulis (a), leaf and LJF (b), caulis and LJF (c), flower and flower bud 4(d).
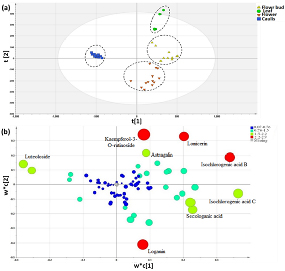
Figure 5. Partial least squares discriminant analysis (PLS‐DA) scores plot(a) loading plot (b) profiles (Green circle represent Leaf samples, Yellow triangles represent Flower bud samples, Orange inverted triangle represent Flower sample, Blue box represent Caulis in Fig. (a); each circle in Fig. (b) represent one compound)
The most common technology for metabolomics is mass spectrometry, and LC-MS is regarded as one of the most applicable and versatile methods in metabolomics, which has been widely used to investigate the metabolic profiles of plant materials. In current study, the differences in metabolic profiles among the aerial parts (including leaf, flower bud, flower, and caulis) of L. japonica were observed by an untargeted LC-MS method. The optimal differential metabolites were selected as chemical markers using PLS-DA analysis, VIP and p-value.
LJF and LJC were the different medicinal parts of L. japonica, they had been documented as independent herbal medicine in Chinese pharmacopeia (2015 Version); However, their traditional efficacy was different. The Venn plot (figure 6) and Table S1 might illustrate this phenomenon by comparing the chemical composition of different parts. Among these 71 metabolites, there were 28 mutual constituents. All the other iridoid compounds in LJC were present except kingside, and the number of iridoid compounds in LJF was less than that in caulis. In general, different compounds possess different pharmacological effects. The biological activities of loganin (one of the optimal chemical markers based on VIP and p-value) including neuroprotection [19,20], antithrombotic and anticoagulation, which were consistent with the efficacy of loganin (the quality marker of LJC) recorded in the Chinese Pharmacopoeia. This analysis might pave the way to elucidating the similarities and differences in the efficacy of LJF and LJC from the perspective of phytochemistry.
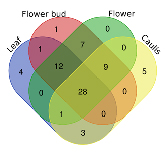
Figure 6. Venn diagram of comparative differential metabolites in the aerial parts. The number in verlapping regions is the amount of intersection of metabolites in the different parts, and the remaining regions shows the specifically metabolites.
Table S1. The metabolites represented by the numbers in the Venn diagram (Figure 6)
|
Names |
Numbers |
Metabolites |
Caulis ∩ Flower ∩ Flower bud ∩ Leaf |
28 |
Rutin, 5-(p-Coumaryl) quinic acid, 3-O-ferulicoylquinic acid, Lonicerin, Valine, Caffeic acid, Hederagenin, Proline, Neochlorogenic acid, Luteolin, Rhoifolin, Hyperoside, 1-O-caffeoylquinic acid, Cryptochlorogenic acid, Secologanic acid, 7-O-ethyl sweroside, Quinic acid, Chlorogenic acid, Vogeloside, Secoxyloganin, Loganic acid, Centauroside, Astragalin, 1,4-O-dicaffeoylquinic acid, Swertiamarin, Sweroside, 1,5-O-dicaffeoylquinic acid, 3-O-caffeoylquinic acid methyl ester |
Flower ∩ Flower bud ∩ Leaf |
12 |
Isoquercitrin, 1,3-O-dicaffeoylquinic acid, Isochlorogenic acid C, 3,4,5-tricaffeoylquinic acid, Kaempferol-3-O-rutinoside, Quercetin, Coumaroyl caffeoylquinic acid isomer, Apigenin, Coumaroyl caffeoylquinic acid, 4,5-O-dicaffeoylquinic acid methyl ester, Isochlorogenic acid A, Isochlorogenic acid B |
Caulis ∩ Flower ∩ Leaf |
1 |
Caffeic acid methyl ester |
Caulis ∩ Flower ∩ Flower bud |
9 |
Diosmetin, Chrysoeriol, Genistein, 8-epi-loganin acid, Akebia saponin D, Dipsacoside B, Flavoyadorinin-B, Loganin, Tricin |
Flower bud ∩ Leaf |
1 |
Secologanin |
Caulis ∩ Leaf |
3 |
Quercetin-7-O-glucoside, Cupressuflavone/Ochanaflavone, Luteolin-5-O-β-D-glucopyranoside |
Flower ∩ Flower bud |
7 |
Kaempferol, Kingiside, Protocatechuic acid, Luteoloside, Hesperidin, Phenylalanine, Feruloyl caffeoylquinic acid |
Leaf |
4 |
Apigenin-7-O-rutinoside, Prunetin, Macranthoidin B, Morroniside |
Flower bud |
1 |
Isoleucine |
Caulis |
5 |
7-epi-loganin, Ferulic acid, 8-epi-loganin, Macranthoidin A, Ethyl caffeate |
Chinese pharmacopeia officially record the best harvest season of LJC was autumn or winter, while the harvest time of LJF is in early summer. This indicates that the harvest time of different TCMs is different, and the harvesting time is indeed one of the factors affecting the quality of TCMs [5,6]. In addition, the chemical composition and content are of crucial importance to the quality, and determine the curative effect in clinical. In order to further explore the effect of harvesting time on the quality of medicinal materials, we compared the chemical compositions of LJF at different harvesting periods (such as flower bud, flower). The result (figure 6) illustrated that the harvesting time had a significant effect on the chemical composition of medicinal materials. And some literatures reported that flower buds have better quality and higher medical value [34,35].
Last but not least, although leaf was not recorded in Chinese pharmacopeia, it could be seen from the figure 6 that the composition of leaves exhibited the most similarity to LJF, which was consistent with the result of previous research [21]. The common components of leaves and LJF include the main bioactivity components: phenolic acid (such as Isochlorogenic acid A, Isochlorogenic acid B, Isochlorogenic acid A), flavonoids (such as Luteolin, Kaempferol-3-O-rutinoside), iridoids (such as Loganin acid) and saponins (such as Hederagenin ).In addition, from the perspective of structure and activity, specific components such as luteolin and kaempferol in flowers can be mutually converted and synthesized with common components such as luteolin and kaempferol-3-rutinoside. While, morroniside is contained in leaves but not in LJF. A large number of published studies has shown that morroniside possess anti-inflammatory, anti-apoptosis, angiogenesis, anti-oxidative stress, neuroprotection and anti-cancer activities [37-38]. These impliy that leaf can be used as an alternative medicinal resource for LJF. This result can provide the support for extending the range of application of L. japonica and reducing the waste of potential resources.
Chemicals, reagents and plant materials
Acetonitrile, methanol, and formic acid (HPLC grade) was supplied by Merck (Darmstadt, Germany). All of other chemicals and reagents were of analytical grade and obtained from Yuanye Biotechnology (Shanghai Yuanye Biotechnology Co., Ltd., China). Ultrapure water was purified with a Milli-Q water purification system (Millipore, Bedford, MA, USA). The authentic standards of chlorogenic acid, neochlorogenic acid, cryptochlorogenic acid, isochlorogenic acid A, isochlorogenic acid B, 1-O-caffeoylquinic acid, ferulic acid, caffeic acid, loganin, Proline,Valine, Isoleucine,Phenylalanine were obtained from Shanghai Yuanye Biotechnology Co. Ltd (Shanghai,China); quinic acid, rutin, astragalin, hyperoside, isoquercitrin were purchased from the Control of Pharmaceutical and Biological Products (Beijing, China); apigenin, diosmetin, kaempferol kaempferol‐3‐O-rutinoside, sweroside, akebia saponin D were provided by Chengdu Chroma Biotechnology Co. Ltd (Sichuan, China); secoxyloganin, dipsacoside B were acquired from Nanjing Jingzhu Biotechnology Co. Ltd (Nanjing, China); 1,3‐O‐dicaffeoylquinic acid, isochlorogenic acid C, protocatechuic acid were received from Chengdu Prefa Technology Development Co. Ltd (Sichuan, China); 4,5‐O-dicaffeoylquinic acid methyl ester, luteoloside, luteolin, rhoifolin lonicerin, secologanic acid, loganin acid, morroniside, macranthoidin A were offered by Liangwei Chemical Reagent Co. Ltd (Nanjing, China). The purity of each compound was more than 98% determined by HPLC analysis.
Forty-four batches of samples (including leaf, flower bud, flower, and caulis) were collected from 4 different provinces in China. Detailed information about these samples is listed in Table 2. The botanical origins of the materials were identified by Professor Xunhong Liu (Department for Authentication of Chinese Medicines, School of Pharmacy, Nanjing University of Chinese Medicine, China). Voucher specimens were deposited in the Herbarium of Pharmacy, Nanjing University of Chinese Medicine.
Table 2. Information on Lonicera japonica Thunb samples
Sample |
No. |
Batch No. |
Habits |
Origin |
Leaf |
S1 |
2.018E+09 |
Henan |
Fengqiu |
|
S2 |
2.018E+09 |
Henan |
Fengqiu |
|
S3 |
2.018E+09 |
Henan |
Fengqiu |
|
S4 |
2.018E+09 |
Henan |
Fengqiu |
|
S5 |
2.018E+10 |
Henan |
Fengqiu |
Caulis |
S6 |
18030825 |
Shandong |
Anhui Dichang Pharmaceutical Co., Ltd. |
|
S7 |
C16052001 |
Jiangsu |
Zhejiang Yedong Pharmaceutical Co., Ltd. |
|
S8 |
180426 |
Jiangsu |
Nantong Sanyue Herbal Medicine Co., Ltd. |
|
S9 |
180501 |
Shandong |
Bozhou Beshixin traditional Chinese Medicine slice Co., Ltd. |
|
S10 |
170601 |
Shandong |
Anhui YaoZhiyuan traditional Chinese Medicine decoction Co., Ltd. |
|
S11 |
180810 |
Shandong |
Ningbo Mingbei Traditional Chinese Medicine Co., Ltd. |
|
S12 |
20170801 |
Shandong |
Local collection |
|
S13 |
170501 |
Shandong |
Local collection |
|
S14 |
20170927 |
Shandong |
Nantong Sanyue Herbal Medicine Co., Ltd. |
|
S15 |
171020 |
Jiangsu |
Shanghai medicine holdings Yixing Co., Ltd. |
|
S16 |
20181101 |
Shandong |
Local collection |
|
S17 |
20181102 |
Shandong |
Local collection |
|
S18 |
20181103 |
Shandong |
Local collection |
|
S19 |
20181104 |
Shandong |
Local collection |
|
S20 |
20181105 |
Shandong |
Local collection |
Flower bud |
S21 |
180701 |
Shandong |
Chongqing Wanli Pharmaceutical Co., Ltd. |
|
S22 |
C16011901 |
Henan |
Zhejiang Yedong Pharmaceutical Co., Ltd. |
|
S23 |
2.018E+09 |
Henan |
Fengqiu |
|
S24 |
20181108 |
Shandong |
Local collection |
|
S25 |
2.018E+09 |
Hebei |
Juluxian Goujijinyinhua market |
|
S26 |
180401 |
Henan |
Anhui YaoZhiyuan traditional Chinese Medicine decoction Co., Ltd. |
|
S27 |
2.018E+09 |
Shandong |
Linyi |
|
S28 |
2.018E+09 |
Henan |
Fengqiu |
|
S29 |
2.018E+10 |
Henan |
Fengqiu |
|
S30 |
2.018E+10 |
Henan |
Fengqiu |
|
S31 |
2.018E+09 |
Henan |
Fengqiu |
Flower |
S32 |
180607 |
Shandong |
Nantong Sanyue Herbal Medicine Co., Ltd. |
|
S33 |
170802 |
Shandong |
Bozhou Beshixin traditional Chinese Medicine slice Co., Ltd. |
|
S34 |
1708021 |
Shandong |
Bozhou Beshixin traditional Chinese Medicine slice Co., Ltd. |
|
S35 |
2.018E+09 |
Shandong |
Linyi |
|
S36 |
2.018E+09 |
Hebei |
Juluxian Gouqijinyinhua market |
|
S37 |
2.018E+09 |
Shandong |
Linyi |
|
S38 |
2.018E+10 |
Hebei |
Juluxian Gouqijinyinhua market |
|
S39 |
2.018E+09 |
Shandong |
Linyi |
|
S40 |
20181107 |
Shandong |
Local herbal medicine market |
|
S41 |
2.018E+09 |
Henan |
Fengqiu |
|
S42 |
171116 |
Jiangsu |
Shanghai medicine holdings Yixing Co., Ltd. |
|
S43 |
2.018E+09 |
Hebei |
Juluxian Gouqijinyinhua market |
|
S44 |
2.018E+09 |
Hebei |
Juluxian Gouqijinyinhua market |
Sample preparation
All of the samples were finely pulverized and sieved through a 50‐mesh. Each dried weighed powder (1.0 g) was soaked in 40 mL of 70% methanol solution in a conical flask. After being shaken violently, the powder was extracted using ultra sonication (500 W, 40 kHz) for 45 min at room temperature; the loss of solvent was replenished by 70% methanol and later centrifuged at 12000 rpm for 10 min (8050 g). The mixture was filtered through a 0.22 μm membrane prior to UFLC-Triple TOF-MS/MS analysis. Finally, the dried samples were stored at -4 ℃ for further analysis.
UFLC-Triple TOF-MS/MS analysis
The UFLC system (SHIMADZUDGU Corp., Kyoto, Japan) with electronic spray ionization (ESI) source was used for sample analysis. In order to obtain a valid, optimal chromatographic condition, various parameters including X Bridge R C18 (Waters, Wexford, Ireland), Agilent ZORBAX SB C18 column (Agilent, Palo Alto, CA, USA) and Thermo Acclaim TM RSLC 120 C18 (Thermo Scientific, Waltham, MA, USA) three types of columns, and water/acetonitrile, water/methanol, 0.1% formic acid aqueous solution/acetonitrile, 0.2% formic acid aqueous solution/acetonitrile four kinds of mobile phases were considered. The result of UFLC indicated that the Agilent ZORBAX SB-C18 column and 0.2% formic acid aqueous solution (A)/acetonitrile (B)were better because of the strong hydrophilicity of organic acids. Meanwhile, the effects of flow rate, temperature, and injection volume were also investigated; In consideration of the baseline, the shape and number of chromatogram peaks, the flow rate of 0.3 mL/min, 35 ℃ of the column temperature and 1 µL injection volume were selected. The gradient elution was optimized and set according to the following schedule: 0–4 min: 2% B; 4–5 min: 2–10% B; 5–25 min: 10-18% B; 25–29 min: 18–25% B; 29–30 min: 25–44% B; 30–33 min: 44–48% B; 33–38 min:48–72% B; 38–41 min: 72–95% B.
Mass spectrometry detection was performed on AB SCIEX Triple TOFTM 5600 System-MS/MS (AB Sciex, Framingham, MA, USA) equipped with an electronic spray ionization (ESI) source in both negative and positive ion mode for the full scan. The full scan mass range was set to m/z 100-2000 to acquire TOF-MS data, the scanning range of m/z 50 to 1500 to acquire TOF-MS/MS. The optimized MS analysis conditions were set as follows: nebulizer gas (GS 1), 55 psi; heater gas (GS 2), 55 psi; curtain gas (CUR), 40 psi; ion spray voltage floating (ISVF), 4500 V; turbo spray temperature (TEM), 550 ℃; declustering potential, -100 V; collision energy, -40 V.
A strategy for comprehensive study on the aerial parts of Lonicera japonica Thunb
In the present work, we developed a strategy integrating metabolic profiling and multi-step PLS-DA analysis to separate the different aerial parts and reveal the chemical markers of L. japonica. (figure 7) There are two main parts to this strategy; one is UFLC-Triple TOF-MS/MS method, which was employed to explore the chemical composition. The other is multi-step PLS-DA, which were applied to distinguish the aerial parts, and to reveal the differential compositions among them, and then selecting the optimal chemical markers.
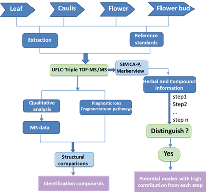
Figure 7. A strategy for comprehensive study the aerial parts of Lonicera japonica Thunb
Data processing
In this study, a database of chemicals from LJF and LJC was established by searching the relevant databases, including Chinese National Knowledge Infrastructure (CNKI), PubMed. Additionally, SciFinder was used to confirm the compound information (chemical names, molecular formulas and structures). The constituents of samples were identified by matching retention time, accurate mass measurement with standard substances and the databases, and rules controlling structural changes of each compound with relevant literature.
The mass spectrometry was collected by the Analyst TF 1.6 software (AB Sciex, USA), the UFLC-Triple TOF-MS/MS data was processed by PeakView1.2 (AB Sciex, USA) and MarkerView 1.2.1 software (AB Sciex, USA). Moreover, PLS-DA was performed on SIMCA-P 13.0 software (for Windows, Umetrics AB, Sweden). URL: http://bioinformatics.psb.ugent.be/webtools/Venn/, which was calculated and drawn custom Venn diagrams.
Principal Component Analysis (PCA) and Partial Least Squares Discriminant Analysis (PLS-DA)
High-dimensional and complex data can be dimensionally reduced to study the characteristics of the metabolic spectrum of samples by multivariate statistical analysis. In this study, uunsupervised Principal Component Analysis (PCA) was performed to elucidate the total metabolic differences among the samples of each group. In order to further investigate the differences among leaf, flower bud, flower, and caulis of L. japonica, the supervised partial least squares discriminant analysis (PLS-DA) and Variable Importance in the Projection (VIP) were carried out.
This study is a portion of a long-term project to explore new strategies for quality evaluation of complex TCMs. This strategy was developed based on the establishment of chemical constituent data sets, metabolic profiling and chemical pattern recognition for leaf, flower bud, flower, and caulis of L. japonica. A total of 71 metabolites were identified from the aerial parts samples. Among them, Lonicerin, Kaempferol-3-O-rutinoside, Loganin, Isochlorogenic acid B, Isochlorogenic acid C, Secologanic acid, Luteoloside, Astragalin were selected as the optimal chemical markers. Eventually, this study laid the foundation for elucidating the differences in efficacy between LJF and LJC at the level of phytochemistry. Meanwhile, from the perspective of the structure-activity relationship, it also implied leaf could be used as an alternative medicinal resource for LJF.
This work was supported by the Priority Academic Programme Development of Jiangsu Higher Education Institutions of China, Numbers: YSXK‐2014.
Zhichen Cai, Xunhong Liu contributed to conception and design, Zhichen Cai Chengcheng Wang, Cuihua Chen, Jiali Chen, Mengxia Tan, Yuqi Mei, Lifang Wei executed the experiments, Zhichen Cai acquisited, analysed of data and wrote the paper, Lisi Zou collected the samples.
The authors declare that they have no conflict of interest.
- Powell KI, Chase JM, Knight TM (2013) Invasive plants have scale-dependent effects on diversity by altering species-area relationships. Science. 339: 316-318. [Crossref]
- Shang X, Pan H, Li M, Miao X, Ding H (2011) Lonicera japonica Thunb.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J Ethnopharmacol. 138: 1-21. [Crossref]
- Chinese Pharmacopoeia Commission. (2015) Pharmacopoeia of the People’s Republic of China. pp. 221, part I. China Medical Science Press, Beijing [Crossref]
- Chinese Pharmacopoeia Commission. (2015) Pharmacopoeia of the People’s Republic of China. pp. 194, part I. China Medical Science Press, Beijing [Crossref]
- Wang S, Hua Y, Lin Y, Zou L, Liu X, et al. (2017) Dynamic changes of metabolite accumulation in Scrophulariae Radix based on liquid chromatography-tandem mass spectrometry combined with multivariate statistical analysis. J Sep Sci. 40: 2883-2894. [Crossref]
- Noémie A, Patrice W, Agnieszka KC, Wilfried A (2018) Bioactive compounds and antioxidant capacity of Lonicera caerulea berries: Comparison of seven cultivars over three harvesting years. J Food Composition and Anal. 66: 81-89. [Crossref]
- Wang S, Hua Y, Zou L, Liu X, Yan Y, et al. (2017) Comparison of Chemical Constituents in Scrophulariae Radix Processed by Different Methods based on UFLC-MS Combined with Multivariate Statistical Analysis. J Chromatogr Sci. 56: 122-130. [Crossref]
- Zhao GM, Li SH, Sun X, Wang YZ, Chang ZP (2015) The role of silicon in physiology of the medicinal plant (Lonicera japonica L.) under salt stress. Sci Rep. 5: 12696. [Crossref]
- Janská A, Maršík P, Zelenková S, Ovesná J (2010) Cold stress and acclimation-what is important for metabolic adjustment. Plant Biology. 12: 395-405. [Crossref]
- Saito MA, McIlvin MR, Moran DM, Goepfert TJ, DiTullio GR, et al. (2014) Multiple nutrient stresses at intersecting Pacific Ocean biomes detected by protein biomarkers. Science. 345: 1173-1177. [Crossref]
- Shi J, Cao B, Wang XW, Aa JY, Duan JA, et al. (2016) Metabolomics and its application to the evaluation of the efficacy and toxicity of traditional Chinese herb medicines. J Chromatogr B Analyt Technol Biomed Life Sci. 1026: 204-216. [Crossref]
- Lee HJ, Suh DH, Jung ES, Park HM, Jung GY, et al. (2015) Metabolomics of Lonicera caerulea fruit during ripening and its relationship with color and antioxidant activity. Food Res Int. 78: 343-351. [Crossref]
- Seo ON, Kim GS, Park S, Lee JH, Kim YH, et al. (2012) Determination of polyphenol components of Lonicera japonica Thunb. using liquid chromatography-tandem mass spectrometry: Contribution to the overall antioxidant activity. Food Chem. 134: 572-577. [Crossref]
- Zhu H, Chen JX, Wen L, Wang ZP, Jing F, et al. (2016) Advances in chemical constituents of lonicera japonica leaves. Shandong sci. 29: 30-39. [Crossref]
- Ma YN, Wang ZY, Guo ZZ, Zhang HY, Zhao TZ. (2017) Determination of total flavonoids in leaves of lonicera japonica. Chin J Exp Trad Med Form. 2355-2359. [Crossref]
- Wang, DJ (2013) Study on chemical constituents of honeysuckle leaves and their resistance to H-5 subtype avian influenza virus. Shandong agricultural university. [Crossref]
- Yan K, Zhao S, Bian L, Chen X (2017) Saline stress enhanced accumulation of leaf phenolics in honeysuckle (Lonicera japonica Thunb.) without induction of oxidative stress. Plant Physiol. Biochem. 112: 326-334. [Crossref]
- Gouveia SC, Castilho PC (2009) Analysis of phenolic compounds from different morphological parts of Helichrysum devium by liquid chromatography with on‐line UV and electrospray ionization mass spectrometric detection. Rapid Commun. Mass Spectrom. 23: 3939-3953. [Crossref]
- Rawji KS, Mishra MK, Michaels NJ, Rivest S, Stys PK, et al. (2016) Immunosenescence of microglia and macrophages: impact on the ageing central nervous system. Brain 139: 653-661. [Crossref]
- Kwon SH, Kim JA, Hong SI, Jung YH, Kim HC, et al. (2011) Loganin protects against hydrogen peroxide-induced apoptosis by inhibiting phosphorylation of JNK, p38, and ERK 1/2 MAPKs in SH-SY5Y cells. Neurochem Int. 58: 533-541. [Crossref]
- Ye J, Su J, Chen K, Liu H, Yan X, et al. (2014) Comparative investigation on chemical constituents of flower bud, stem and leaf of Lonicera japonica Thunb. by HPLC-DAD-ESI-MS/MS n and GC-MS. J anal Chem. 69: 777-784. doi:10.1134/s1061934814080036 [Crossref]
- Zhang YD, Huang X, Zhao FL, Tang YL, Yin L. (2015) Study on the chemical markers of Caulis Lonicerae japonicae for quality control by HPLC-QTOF/MS/MS and chromatographic fingerprints combined with chemometrics methods. Anal Methods. 7: 2064-2076. [Crossref]
- Li PL, Li CY, Liu MH, Wang DQ, Bai Y, et al (2016) Chemical composition comparison of LJF and LF based on UFLC-Triple- Q-TOF-MS/MS. Central pharmacy. 363-369. [Crossref]
- Zhou J, Yi H, Zhao ZX, Shang XY, Zhu MJ, et al. (2018) Simultaneous qualitative and quantitative evaluation of Ilex kudingcha CJ tseng by using UPLC and UHPLC-qTOF-MS/MS. J pharm and biomed. Anal. 155: 15-26. [Crossref]
- Kucharska AZ, Sokół-Łętowska A, Oszmiański J, Piórecki N, Fecka I (2017) Iridoids, phenolic compounds and antioxidant activity of edible honeysuckle berries (Lonicera caerulea var. kamtschatica Sevast.). Molecules. 22: 405. [Crossref]
- Ding G, Wang Y, Liu A, Hou Y, Zhang T, et al. (2017) From chemical markers to quality markers: an integrated approach of UPLC/Q-TOF, NIRS, and chemometrics for the quality assessment of honeysuckle buds. RSC Advances. 7: 22034-22044. [Crossref]
- Li Z, Guo X, Cao Z, Liu X, Liao X, et al. (2018) New MS network analysis pattern for the rapid identification of constituents from traditional Chinese medicine prescription Lishukang capsules in vitro and in vivo based on UHPLC/Q-TOF-MS. Talanta. 189: 606-621. [Crossref]
- Song Y, Li SL, Wu MH, Li HJ, Li P (2006) Qualitative and quantitative analysis of iridoid glycosides in the flower buds of Lonicera species by capillary high performance liquid chromatography coupled with mass spectrometric detector. Analytica Chimica Acta. 564: 211-218. [Crossref]
- Qi LW, Chen CY, Li P (2009) Structural characterization and identification of iridoid glycosides, saponins, phenolic acids and flavonoids in Flos Lonicerae Japonicae by a fast liquid chromatography method with diode‐array detection and time‐of‐flight mass spectrometry. Rapid Commun Mass Spectrom. 23: 3227-3242. [Crossref]
- Ye J, Su J, Chen K, Liu H, Yang X, et al. (2014) Comparative investigation on chemical constituents of flower bud, stem and leaf of Lonicera japonica Thunb. by HPLC-DAD-ESI-MS/MS n and GC-MS. J anal Chem. 69: 777-784. [Crossref]
- Yin LL (2007) The cleavage of flavonoids was studied by negative ESI-IT-TOF/MS. Chinese pharmaceutical association: Reports and proceedings of the ninth national symposium on traditional Chinese medicine and natural medicine 10. [Crossref]
- Fabre N, Rustan I, Hoffmann E, Quetin-Leclercq J (2001) Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J the Amer Soci for Mass Spec. 12: 707-715. [Crossref]
- An GY, Chung SW, Cho HC, Park JR , Kim MJ, et al. (2018) Phytochemical Constituents of Lonicera maackii Stems. Korean J Pharmacognosy. 49: 103-107. [Crossref]
- Cai ZC, Wang CC, Chen CH, Zou LS, Chai C, et al. (2019) Quality evaluation of Lonicerae Japonicae Flos and Lonicerae Flos based on simultaneous determination of multiple bioactive constituents combined with multivariate statistical analysis. Phytochem Anal. 1-12. [Crossref]
- Yuan Y, Song L, Li M, Liu G, Chu Y, et al. (2012) Genetic variation and metabolic pathway intricacy govern the active compound content and quality of the Chinese medicinal plant Lonicera japonica thumb. BMC genomics. 13: 195-212. [Crossref]
- Liu T, Xiang B, Guo D, Sun FL, Wei RP, et al. (2016) Morroniside promotes angiogenesis and further improves microvascular circulation after focal cerebral ischemia/reperfusion. Brain research bulletin. 127: 111-118. [Crossref]
- Zhang JX, Wang R, Xi J, Lin S, Zhu AY, et al. (2017) Morroniside protects SK-N-SH human neuroblastoma cells against H2O2-induced damage. International j of molecular med. 39: 603-612. [Crossref]
- Yu B, Wang W (2018) Cardioprotective effects of morroniside in rats following acute myocardial Infarction. Inflammation. 41: 432-436. [Crossref]









