Background: Tocilizumab is a recombinant humanized anti-interleukin 6 (IL-6) receptor monoclonal antibody that inhibits the binding of IL-6 to both membrane and soluble IL-6 receptors, blocking thereby IL-6 signaling and dampening inflammation. Although it has been the second drug ever recommended by the world health organization for Covid-19 treatment after recommending dexamethasone, the role of IL-6 inhibition in reducing Covid-19 severity and mortality remains controversial since several large-scale, multi-center observations and randomized controlled trials have shown minimal benefits whereas others such as the REMAP-CAP and Recovery trials have suggested that tocilizumab in combination with systemic corticosteroids would be expected to improve outcomes in hospitalized covid-19 patients. Herein, we report a hospital-based prospective monocentric study of a series made up of 22 adult patients who received tocilizumab out of a total of 139 patients admitted to the medical intensive care unit for moderate to severe forms of Covid-19 between February 2021 and October 2021.
Objective: The aim of this study is to assess the impact of tocilizumab on clinical outcomes mainly on oxygen needs and mortality along with summarizing the available evidence regarding its efficacy in hospitalized covid-19 patients.
Location: The study was conducted in the medical intensive care unit in the Ibn Rochd University Hospital of Casablanca, Hassan 2 University of Casablanca, Morocco.
Participants: The study consisted of a series of 22 adult patients out of a total of 139 patients admitted to the unit between February 1,2021 and October 7,2021 for moderate, severe, or critical form of Covid-19. Recruitment of patients was based upon inclusion and exclusion criteria.
Intervention: Tocilizumab with concomitant systemic corticosteroids along with usual standard of care.
Main measurements: Oxygen requirements and overall mortality. The study focused on the impact tocilizumab has on reducing oxygen needs and therefore improving respiratory status and mortality.
Results: A beneficial effect of the combination tocilizumab and systemic corticosteroids on oxygen needs and mortality has been suggested by the study (P=0,008).
Conclusions: An early combined use of tocilizumab and systemic corticosteroids may improve clinical outcomes and overall mortality in hospitalised Covid-19 patients. Further randomized controlled trials would therefore be of paramount to pinpoint the subgroups that are most likely to benefit from tocilizumab treatment.
Sars-cov2; Tocilizumab; Outcomes; Intensive care
Hyper-inflammatory response induced by severe acute respiratory syndrome coronavirus 2 (Sars-cov2) is a major cause of disease severity and death. Several therapeutic agents have been studied to assess their efficacy in reducing mortality rates and improving outcomes in hospitalized patients [1]. One of these agents includes tocilizumab, an interleukin (IL)-6 inhibitor commonly used for autoimmune diseases such as refractory rheumatoid arthritis [2] and cytokine release syndrome (CRS) [3,4]. The theory behind their potential benefit in Covid-19 is the pivotal role of cytokine dysregulation in critical Covid-19 cases with IL-6 the key driver of this hyperinflammation [5]. However, randomized controlled trials of tocilizumab in Covid-19 have so far shown mixed results for 28-day mortality. The purpose of this study is to assess the effect of tocilizumab on clinical outcomes chiefly on oxygen needs and mortality along with summarizing the available evidence regarding its efficacy in hospitalized covid-19 patients. Therefore, the aims of the study might have a significant clinical importance in daily practice as they would allow a more protocolized use of Tocilizumab in hospitalized covid-19 patients.
Study Design and Settings: The study has been designed as a hospital-based prospective observational monocentric study carried out in the medical intensive care unit of the Ibn Rochd university hospital of Casablanca consisting of a series of 22 adult patients who received tocilizumab out of a total of 139 patients admitted to the unit between February 1,2021 and October 7,2021 for moderate, severe, or critical form of Covid-19.
Study Participants and Recruitment: Patients allocated to tocilizumab were to receive a single intravenous infusion of 400mg over 3 hours, as soon as it was indicated according to a local protocol set by the expert committee of the Ibn Rochd university hospital of Casablanca. The protocol comprises three fundamental components that must be met in order that tocilizumab could be administered:
- No hepatic cytolysis.
- Normal procalcitonin level (PCT) < 0,5 µg/L.
- IL6 serum level more than three times the upper normal limit (IL6 > 21 UI/L).
All eligible patients who were assigned to tocilizumab received concomitant systemic corticosteroids as well (1mg/kg per day of Methylprednisolone over 5 days), along with usual standard of care. At the time of administration of IL6-inhibitor, patients were either receiving oxygen support (oxygen mask and/or high-flow nasal oxygen) or under non-invasive ventilation. No patient was under invasive mechanical ventilation at the time of administration of tocilizumab. Recruitment of patients was based upon inclusion and exclusion criteria. Inclusion criteria included a Positive Covid-19 RT-PCR, Patients hospitalized for moderate, severe, or critical form of Covid-19, an increase in oxygen needs, no hepatic cytolysis, Procalcitonin (PCT) level < 0,5 µg/L, IL-6 serum level > 21 UI/L. Patients with evidence of hepatic dysfunction, active tuberculosis infection, or clear evidence of active bacterial (PCT> 0,5 µg/L.), fungal, viral, or other infection (besides Covid-19) were not eligible for tocilizumab. Likewise, Patients who were under invasive mechanical ventilation were not allocated to tocilizumab.
Data Collection and Analyses: clinical and paraclinical data were collected and measured using bedside monitoring as well as laboratory tests. Quantitative and qualitative variables as well as subgroups interactions have been handled and analyzed using Excel software and presented with percentages and figures. Missing data have not been included in the investigation to avoid any potential source of bias. Data entry was done using Excel software, and analysis was performed using SPSS version 16 software. A univariate descriptive analysis was carried out where we calculated proportions, numbers, mean and standard deviations (S.D). A bivariate analysis was performed using the paired –samples t-test to compare means one day before and three days after tocilizumab infusion. A p-value ≤ 0.05 was considered significant.
Of the 139 patients admitted to the medical intensive care unit with moderate, severe, or critical forms of Sars-cov2 pneumonia during 2021; tocilizumab was indicated in 22 patients according to the local Covid-19 management protocol. Twelve patients, 54.5%, were male and 10 patients,45.5%, were female. The mean age was 66 years old with extremes ranging from 47 to 88 years old (Table 1).
Table 1. Sociodemographic features of patients
Sociodemographic features (Gender/Age) |
Number |
Percentage |
Male |
12 |
54.5% |
⩾65 years old |
13 |
60% |
Twelve patients, around 54.5% had type 2 diabetes. Seven patients, just under 32%, had been being treated for high blood pressure. Morbid obesity was noted in one patient. Ischemic cardiopathy was also noted in one patient. Four patients, 18.2%, were chronic smokers. On admission to the intensive care unit (Table 2), all patients were conscious 15/15 without any sensory-motor deficit.
Table 2. Clinical and paraclinical features of patients upon admission
Clinical/Paraclinical features |
Minimal |
Maximal |
Mean |
Glasgow coma scale (upon admission) |
15 |
15 |
15 |
SPO2 (%) (room air) |
60 |
92 |
76 |
Respiratory rate (cpm) |
16 |
40 |
26 |
MAP: Mean arterial pressure (mmhg) |
⩾65 |
⩾65 |
⩾65 |
Temperature (°C) |
36 |
39 |
37 |
C-reactive protein (mg/l) |
43 |
336 |
166 |
White cells (per mm3) |
4470 |
26100 |
11140 |
Lymphocytes (per mm3) |
430 |
1670 |
953 |
Interleukin-6 (IL-6) (IU/l) |
28 |
768 |
148 |
The mean oxygen blood saturation was 76% in room air with extremes varying from 60 to 92%. The mean respiratory rate was 26 cycles per minute with extremes ranging from 16 to 40 cycles per minute. Sixteen patients, nearly 72%, showed signs of acute respiratory failure with paradoxical breathing noted in 13.6%. All patients were, however, hemodynamically stable upon admission. Three patients, around 13%, were febrile. The initial chest CT scan performed upon admission showed more than 50% of parenchymal involvement in 86.4% of cases (Table3).
Table 3. Parenchymal involvement in chest CT scan upon admission
|
⩾50% of interstitial involvement |
<50% of interstitial involvement |
Chest CT Scan (parenchymal involvement) |
86.4% |
13.6% |
Biologically, the mean CRP level was 166 mg/L with a minimum of 43 and a maximum of 336 mg/L. Ten patients, roughly 45%, had neutrophilic leukocytosis with a maximal of 26100/mm3. Seventeen patients, around 77%, had lymphopenia with an average of 809/mm3. Procalcitonin was negative in all patients. Interleukin 6 (IL-6) serum level was more than three times the upper normal limit in all patients with 148 IU/l as a mean serum level, ranging from 28 to 768 IU/l. The rest of the biological work-up chiefly serum ionogram and hemostasis showed no particularities. The initial management consisted of non-invasive oxygen support in all patients using a high concentration mask in 20 patients ,90%, and nasal cannula in two patients. The average oxygen flow rate to maintain a SPO2 ≥ 92% fluctuated around 12 l/min with extremes ranging from 2 to 15 l/min. Venous thromboprophylaxis was assured by curative anticoagulation using low molecular weight heparin (Bemiparin sodium) in 86% and standard heparin in 14%. Empiric antimicrobial therapy, subsequently adapted to antibiogram, was assigned to all patients. A wide range of Antibiotics had actually been used such as 3rd generation cephalosporins, mainly Ceftriaxone and Ceftazidime; fluoroquinolones like Ciprofloxacin and Moxifloxacin; carbapenems such as Imipenem; aminoglycosides: Amikacin and Gentamycin; glycopeptides like Vancomycin and Teicoplanin; as well as Piperacillin-Tazobactam. Systemic corticosteroid therapy, 1mg/kg per day of methylprednisolone over 5 days, as well as platelet anti-aggregation therapy based upon acetyl salicylic acid were assigned to all patients. Tocilizumab was administered as soon as it was indicated according to the local Covid-19 management protocol, with an average of 3 days after admission. The effect of tocilizumab on oxygen needs was assessed from day 3 of administration owing to its mean onset of action of around 72 hours. This effect was statistically significant with a P value=0.008. Indeed, 59% of patients increased their oxygen needs requiring non-invasive ventilation with a progressive rise in inspiratory support, positive end-expiratory pressure, and inspired oxygen fraction; ending up in invasive mechanical ventilation. This group of patients had poor outcomes (Figure 1).
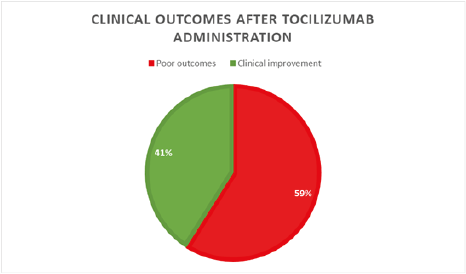
Figure 1: Pie chart displaying the evolution of patients after Tocilizumab infusion
Nevertheless, 41% of patients had a marked decrease in their oxygen requirements with a progressive reduction in oxygen flow until definitive weaning. The prognosis was favorable in this group of patients (Figure 1). Statistical data analysis of the group of patients who had not responded to tocilizumab administration showed that 62% were female and over 60% were 65 years old or older. Furthermore, around 23% were diabetic and just under 10% had high blood pressure while roughly 38% combined hypertension and type 2 diabetes. One patient had ischemic heart disease. Just under 25% of patients had no cardiovascular risk factors, though (Figure 2).
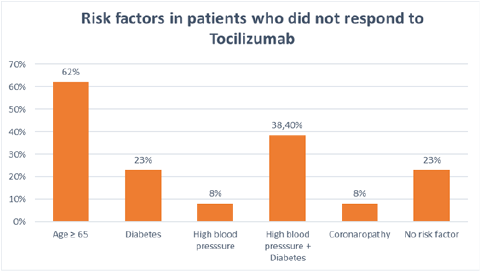
Figure 2: Bar chart representing risk factors in patients who did not respond to Tocilizumab
In addition, over 90% of patients had more than 50% of parenchymal involvement on chest CT scan (Figure 3). CRP serum level was >100mg/l in all patients, and >200mg/l in 54%, with an average of 198 mg/l (Figure 4). Lymphopenia < 1500/mm3 was noted in 84% of patients, of whom 61% had lymphopenia < 1000/mm3; mean lymphopenia was 912/mm3. Serum interleukin 6 levels were >100 IU/L in approximately 70% of cases with an average of 191 IU/L (Figure 5). Regarding the timing of tocilizumab administration, 62% received the infusion after 48 hours of admission while it should be emphasized that over 55% of patients who responded to tocilizumab received their infusion within the first 48 hours of admission.
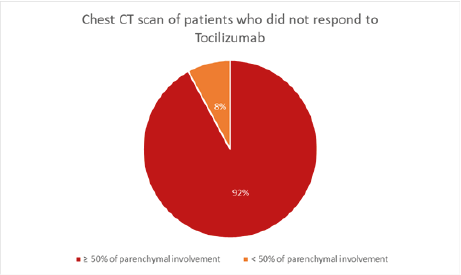
Figure 3: Pie chart depicting parenchymal involvement in the chest CT scan of patients who did not respond to Tocilizumab
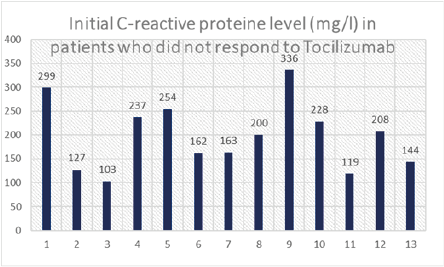
Figure 4: Bar chart displaying initial CRP levels in patients who did not respond to Tocilizumab
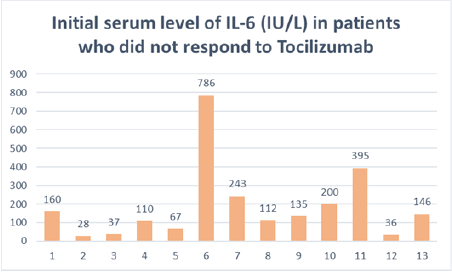
Figure 5: Bar chart showing initial IL-6 serum levels in patients who did not respond to Tocilizumab
Tocilizumab is a monoclonal antibody that acts as an antagonist of the IL-6 receptor (IL-6R). Production of endogenous IL-6, a pleiotropic cytokine, is induced by inflammatory stimuli and mediates a variety of immunological responses that could stimulate a hyperinflammatory state, which might lead to increased alveolar-capillary blood-gas exchange dysfunction resulting in acute respiratory distress syndrome (ARDS). IL-1β and TNFα, in particular, are the main activators of IL-6 expression [6]. Under normal circumstances, the levels of IL-6 are very low, and are increased when there is an infection or insult. Excessive release of IL-6 can cause a cytokine storm, with levels of IL-6 found to be proportional to the severity of the cytokine release syndrome (CRS) [7]. Targeting IL-6 has become a point of interest in the treatment of Covid-19, as it has been found to be the most significant predictor of disease progression and mortality in patients [8]. Although the exact mechanism of action of tocilizumab is still unclear, the main hypothesis relies on tocilizumab preventing IL-6 from binding to its receptor and promoting the CRS sometimes seen in severe Covid-19 which leads to life-threatening multiorgan damage [9]. In short, the IL-6/IL-6R complex binds to the signal transducer glycoprotein 130 (gp130) and the downstream signal is mediated by JAK/STAT3. However, IL-6R exists in two forms: membrane-bound (mIL-6R) and soluble form (sIL-6R). IL-6 binds to mIL-6R, which is predominantly expressed on immune cells, and activates the cis-signaling pathway that promotes lymphocytes activation and differentiation. When IL-6 binds to sIL-6R, which is found virtually on all endothelial cells, the trans-signaling pathway is activated leading to increased vascular leakage and permeability, recruitment of neutrophils and monocytes and importantly, an increase in systemic cytokines production that leads to a cytokine storm [10, 11]. Tocilizumab is able to bind to mIL-6R and sIL-6R inhibiting both the cis and trans-signaling pathways, potentially treating or even preventing the cytokine storm. It is worth noting, however, that tocilizumab has a non-linear pharmacokinetic profile, with a dose-response curve that plateaus at an approximate dose of 800 mg [12]. The half-life of tocilizumab is dose-dependent and comparable to the half-life of IgG1 [13]. Eight randomized trials all assessed as at low risk of bias compared tocilizumab with usual care or placebo in hospitalized patients with Covid-19. Of these trials, only the REMAP-CAP trial in critically ill patients found a significant reduction in 28-day mortality with tocilizumab. A meta-analysis of these eight trials, which included a total of 439 deaths among 2379 patients showed no significant difference in 28-day mortality (death rate ratio 0·89, 95% CI 0·72–1·11) [14]. The RECOVERY trial, however, the largest randomized trial of the effect of tocilizumab in hospitalized patients with Covid-19, found that in 4116 Covid-19 patients with hypoxia and a raised C-reactive protein, tocilizumab reduced 28-day mortality, increased the probability of discharge within 28 days, and, among patients who were not receiving invasive mechanical ventilation at randomization, reduced the probability of progression to the composite outcome of invasive mechanical ventilation, renal replacement therapy, and death [14]. When all these nine trials are considered together, allocation to tocilizumab is associated with a significant 14% proportional reduction in 28-day mortality [14]. Nonetheless, Analyses of these data suggests that trials using tocilizumab monotherapy showed no clinical benefit. With systemic corticosteroids becoming part of standard care in severe Covid-19 [15], the combined use of tocilizumab and corticosteroids in later trials showed a decrease in mortality and hospitalization among other clinical benefits as seen in the REMAP-CAP and RECOVERY trials [6]. The RECOVERY trial in particular showed mortality benefit with tocilizumab only in patients who received concomitant systemic corticosteroids [6]. While REMAP-CAP showed the greatest benefits in patients with the highest C-reactive protein levels (CRP), and the RECOVERY trial recruited only patients with a CRP > 75 mg/dL, the true predictive nature of CRP still requires further investigation. Overall, in hospitalized patients with severe Covid-19, who are hypoxic and have a CRP ≥ 75 mg/L, the current evidence suggests that the combination of tocilizumab and corticosteroids reduces mortality [6]. Whether hypoxic patients with a CRP of less than 75 mg/L could benefit from tocilizumab is still unknown [14] and all our patients had a CRP level > 100 mg/l. Another meta-analysis [16] published on May 18,2021 included 25 peer-reviewed publications with more than 10201 individuals to analyze the correlation of tocilizumab with clinical outcomes among Covid-19 patients. The meta-analyses stratified sub-groups revealing that disease severity, age, and sex play important roles in determining the efficacy of tocilizumab. According to our analysis, a statically significant correlation (P=0,008) of tocilizumab with clinical outcomes has been found. The association is even stronger among moderate to severe Covid-19 patients and published prospective randomized clinical trials back our conclusion suggesting that Covid-19 patients with moderate or severe disease were more likely to benefit from tocilizumab [17]. Furthermore, more than 60% of patients who did not respond to tocilizumab were female and had more than 65 years old; and more than one third associated at least two underlying comorbidities including high blood pressure and diabetes. The randomized placebo-controlled COVACTA trial included patients with different coexisting comorbidities with a disease that ranged from moderate hypoxia to invasive mechanical ventilation at baseline whereas the EMPACTA trial enrolled less severe patients who were not receiving mechanical ventilation at baseline and were at an earlier disease stage [18]. In the former, the median time to hospital discharge was 20 days in the tocilizumab group and 28 days in the placebo group versus 6.0 and 7.5 days respectively in the latter. The results of these trials suggest that patients who are most likely to benefit from tocilizumab are those with moderate to severe disease (i.e., they have hypoxia but are not yet receiving mechanical ventilation) and that tocilizumab may add to the potential benefits of glucocorticoids in hospitalized Covid-19 patients. However, there was no significant difference in the incidence of death from any cause in both trials. Another point to stress is that most of our patients received tocilizumab quickly after admission to intensive care, which presumably represents a hyperinflammatory prime and several other multi-center cohort studies revealed a correlation of early tocilizumab administration with lower mortality rates among critically ill Covid-19 patients with a rapid disease trajectory [19]. Regarding the timing of drug administration, which may therefore potentially affect treatment outcomes, a pre-print study evaluated the effect of early versus late administration of the drug on the outcomes. The authors found that for each additional day of delay from the admission to tocilizumab administration, the odds of receiving mechanical ventilation independently increase by 21% (95% CI: [1.08, 1.38], p = 0.002) [20]. Last but not least, it is worth remembering that clinical studies on patients with Covid-19 have also evoked some safety concerns. The risk of secondary infection complications is still unclear. Among the studies with a comparison group, six found a higher rate of infections among treated, compared to untreated, patients [21-26]. However, since complicating bacterial infections are infrequent in the early hospitalization period of Covid-19, this recognized concern in relation to the use of tocilizumab would be lessened with earlier use [27]. Hepatotoxic effects, neutro-and thrombocytopenia as well as intestinal perforation have also been described [28-30]. This is worrying if considering the mixed results of the randomized controlled trials particularly in patients not requiring mechanical respiratory support, when treated with tocilizumab versus controls.
Although the efficacy of interleukin-6 receptor blockade in hospitalized Covid-19 patients who are not receiving mechanical ventilation remains unclear, several randomized controlled trials have shown promising clinical benefits of the concomitant use of tocilizumab and systemic corticosteroids as did the REMAP-CAP and RECOVERY trials. Likewise, and since all our patients received corticosteroids, a statically significant effect (P=0,008) of the early combined use of tocilizumab and corticosteroids on clinical outcomes has been shown in our study. More large randomized controlled trials are therefore desperately needed to point out the subpopulations of Covid-19 patients that are most likely to benefit from tocilizumab treatment.
Funding: The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Competing interests: The authors have no relevant financial interests to disclose.
Data availability: The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval: This is an observational study. The Research Ethics Committee has confirmed that no ethical approval is required.
Consent to participate: informed consent was obtained during data collection.
Consent to publish: The manuscript contains no individual personal details, images, nor videos.
CRediT author statement: Pr Boubaker Charra: Conceptualization, Methodology, Validation, Resources, Supervision, Project administration. Dr Yassine Bou-ouhrich: Methodology, Investigation, Writing Original Draft, Writing Review&Editing, visualization. Dr Sanaa Ameayou: Software, formal analysis, Data curation. Pr samira Hassoune: Formal analysis, Validation, Data curation.
We thank the department of Clinical Epidemiology for assistance with statistical analysis. We thank the staff of the medical intensive care unit of the Ibn Rochd University Hospital for their contribution to the study.
The authors declare no conflict of interest.
- Ali MJ., Hanif M., Haider MA (2020) Treatment options for COVID-19 : a review. Front Med (Lausanne) 7: 480. [Crossref]
- Han Q., Guo M., Zheng Y (2020) Current evidence of interleukin-6 signaling inhibitorsin patients with COVID-19 : a systematic review and meta-analysis. Front Pharmacol 11: 615972. [Crossref]
- Shimabukuro-Vornhagen, A., Gödel, P., Subklewe, M (2018) Cytokine release syndrome. j. immunotherapy cancer 6: 56
- Le RQ., Li L., Yuan W., Shord SS., Nie L., et al. (2018) FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. Oncologist 23: 943-947. [Crossref]
- Atal S., Fatima Z (2020) IL-6 inhibitors in the treatment of serious COVID-19: a promising therapy? Pharmaceut Med 34: 223-231. [Crossref]
- Walid Alam., Abdul Rahman (2021) Efficacy of tocilizumab in COVID-19: A review of the current evidence. Sci Prog 104: 1-19. [Crossref]
- Zhang S., Li L., Shen A., Chen Y., Qi Z (2020) Rational Use of Tocilizumab in the Treatment of Novel Coronavirus Pneumonia. Clin Drug Investig 40 : 511-518. [Crossref]
- Santa Cruz A., Mendes-Frias A., Oliveira AI., Dias L., Matos AR., et al. (2021) Interleukin-6 Is a Biomarker for the Development of Fatal Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia. Front Immunol 12 : 613422. [Crossref]
- Cortegiani A., Ippolito M., Greco M., Granone V., Protti A., et al. (2020) Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review. Pulmonology 27: 52-66. [Crossref]
- Zegeye, MM., Lindkvist, M., Fälker, K (2018) Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Commun Signal 16: 55. [Crossref]
- Moore, JB., June, CH (2020) Cytokine release syndrome in severe COVID-19. Science 368: 473-474. [Crossref]
- Sheppard M., Laskou F., Stapleton PP., Hadavi S., Dasgupta B (2017) Tocilizumab (actemra). Hum Vaccines Immunother 13: 1972-1988. [Crossref]
- Nishimoto N., Mima T. Tocilizumab (2009) In: Rheumatoid Arthritis. Elsevier Inc.
- RECOVERY Collaborative Group (2021) Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 397: 1637-1645. [Crossref]
- World Health Organization (2021) Corticosteroids for COVID-19.
- Wei Q., Lin H., Wei RG., Chen N., He F., et al. (2021) Tocilizumab treatment for COVID-19 patients: a systematic review and meta-analysis. Infect Dis Poverty 10: 71. [Crossref]
- Salama C., Han J., Yau L., Reiss WG., Kramer B., et al. (2021) Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med 384: 20-30. [Crossref]
- Rosas I., Bräu N., Waters M (2020) Tocilizumab in hospitalized patients with COVID-19 pneumonia. [Crossref]
- Stone JH., Frigault MJ., Serling-Boyd NJ., Fernandes AD., Harvey L., et al. (2020) Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med 383: 2333-2344. [Crossref]
- Petrak R., Skorodin N., Van Hise NW., Fliegelman RM., Pinsky J,Didwania V (2020) Tocilizumab as a therapeutic agent for critically Ill patients infected with SARS-CoV-2. medRxiv. [Crossref]
- Campochiaro C., Della-Torre E., Cavalli G., De Luca G., RipaM, Boffini N (2020) Efficacy and safety of tocilizumab in severe COVID-19 patients : a single-centre retrospective cohort study. Eur J Intern Med 76: 43-49. [Crossref]
- Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R,Menozzi M (2020) Tocilizumab in patients with severe COVID19: a retrospective cohort study. Lancet Rheumatol. [Crossref]
- Quartuccio L., Sonaglia A., McGonagle D., Fabris M., Peghin M,Pecori D (2020) Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: results from a single Italian Cen tre study on tocilizumab versus standard of care. J Clin Virol. [Crossref]
- Ip A., Berry DA., Hansen E., Goy AH., Pecora AL., et al. (2020) Hydroxychloroquine and tocilizumab therapy inCOVID-19 patients-an observational study. medRxiv. [Crossref]
- Kimmig LM., Wu D., Gold M., Pettit NN., Pitrak D., et al. (2020) IL6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections. medRxiv. [Crossref]
- Somers EC., Eschenauer GA., Troost JP., Golob JL., GandhiTN., et al. (2020) Tocilizumab for treatment of mechanically ventilated patients with COVID-19. medRxiv. [Crossref]
- Baskaran V., Lawrence H., Lansbury L (2020) Co-infection in critically ill patients with COVID-19: an observational cohort study from England. medRxiv. [Crossref]
- EMA (2020) RoActemra - Summary of Product Characteristics.
- Fda (2020) ACTEMRA - HIGHLIGHTS OF PRESCRIBINGnINFORMATION.
- Sepriano A., Kerschbaumer A., Smolen JS., van der Heijde D., Dougados M., et al. (2020) Safety of synthetic and biological DMARDs: a systematic literature review informing the2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 79:760-770. [Crossref]





