The increased deposition of iron in gastric mucosa is known as gastric siderosis. We describe a case of a 69-year-old woman with dyspepsia, who had previously consumed oral ferrous sulfate tablets. Gastroscopy revealed inflammation in the gastric antrum. Histologically, there was evidence of mild chronic gastritis and Perl’s stain positive for iron. Iron studies were unremarkable. There have been three distinct patterns of histology identified in the literature for gastric siderosis. This case is unique given the combination of histopathological crossover between two different patterns.
Gastric Siderosis; Iron Tablets; Iron Overload
Iron is predominantly used in the production of hemoglobin and its uptake mainly occurs in duodenal enterocytes [1]. Oral iron supplementation with inorganic iron compounds is the most widely used treatment for iron deficiency [2]. It is generally well-tolerated however side effects can include nausea, abdominal pain, constipation, and dark stools. Rare adverse effects include chronic gastritis and deposition of iron in the gastric mucosa, known as gastric siderosis. If unrecognised, this could potentially lead to mucosal necrosis and ulceration [3]. Herein, we report a case of incidental finding of gastric siderosis in the workup of dyspepsia.
A 69-year-old Caucasian woman was referred by her general practitioner to the gastroenterology clinic in September 2021 for evaluation of dyspepsia.
She experienced progressively worsening dyspepsia for the last 7 years for which she took over the counter antacid tablets. Past medical history was significant for chronic obstructive pulmonary disease. She previously had iron deficiency and took ferrous sulfate tablets intermittently for six months. Her last consumption of iron was a year prior and never had previous blood transfusions. Apart from inhalers, she did not take any other regular medications including non-steroidal anti-inflammatory drugs. She denied abdominal pain, nausea, vomiting, diarrhoea, constipation, dysphagia, weight loss, arthralgia, palpitations, or skin deformities. She smokes 15 cigarettes per day since the age of 20 and occasionally drinks alcohol. She had no significant family history and no previous endoscopic procedures. Her vital signs were within normal limits and physical examination was unremarkable.
Initial baseline full blood count, urea, electrolytes, and liver function tests in June 2021 were unremarkable (hemoglobin of 140 g/L). Iron studies were also unremarkable (total iron 13 umol/L, total iron binding capacity 52 umol/L, iron saturation 25%, and ferritin 134 ug/L). Electrocardiogram and chest X-ray were normal. There was no dedicated imaging of the abdomen performed.
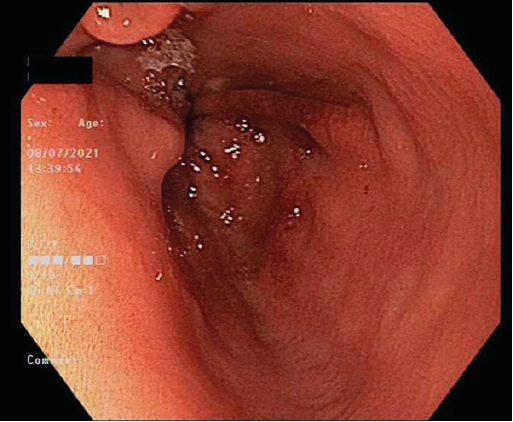
Figure 1: Esophagogastroduodenoscopy. Endoscopic appearance of gastric antrum revealing inflammation
She proceeded to have an esophagogastroduodenoscopy (EGD) which revealed a small hiatus hernia and inflammation in the gastric antrum, with a normal oesophagus and duodenum (Figure 1). Gastric body biopsies revealed mild chronic gastritis, however gastric antrum histology revealed reactive gastropathy with brown pigment and Perl’s stain positive for iron (Figure 2A-C). Helicobacter-like organisms were not identified.
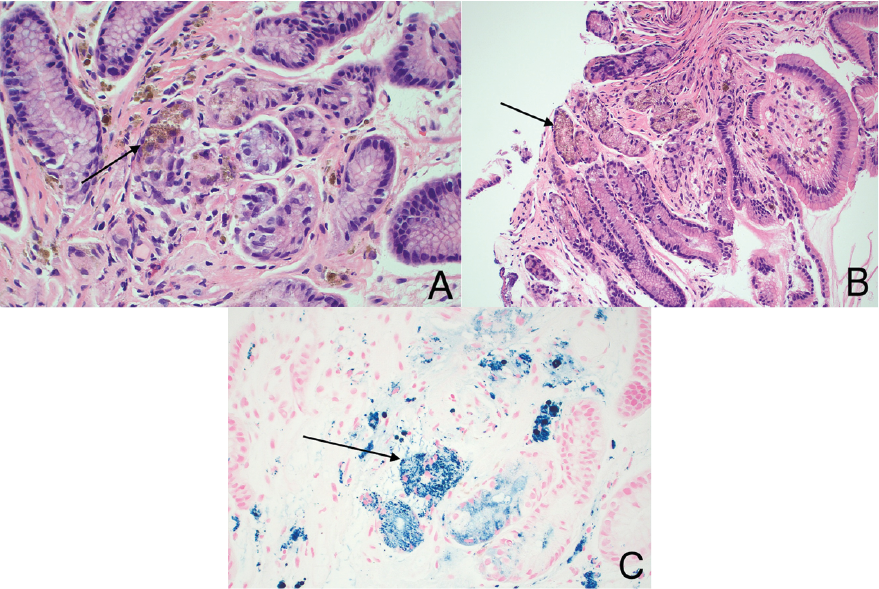
Figure 2. Gastric biopsy. A-C: Light microscopic examination of the gastric biopsy showing mild chronic gastritis with brown pigment and Perl’s stain positive for iron. H&E 20x
The patient was diagnosed with incidental finding of gastric glandular siderosis. She was prescribed regular proton pump inhibitor with good effect and remained off oral iron supplements. At follow up 4 months later, her iron studies remained normal.
Iron is a ubiquitous trace element found within the human body and used in the production of hemoglobin and myoglobin [4]. Dietary iron is absorbed through protein channels on enterocytes within the duodenum [1, 5]. Reduced iron in its soluble form (Fe2+) binds to divalent metal transporter-1, translocates across the apical surface of enterocytes, then exits through the basolateral surface to enter circulation [1, 5, 6]. It is then re-oxidised to Fe3+ and travels via transferrin to various sites of the body including liver parenchymal cells for storage as ferritin, or erythroid marrow for use in erythropoiesis depending on the body’s iron demand [1, 6-8]. Furthermore, iron left in its ferrous or ferric form are catalysts for the formation of reactive oxygen species and free radical production causing direct mucosal injury and organ damage [9].
Once the body’s iron demand is low, iron can be stored as ferritin or hemosiderin in the liver, however if iron absorption exceeds demand, it is stored in other sites including joints, heart, pancreas, stomach or thyroid tissue [5, 8, 10]. The stomach has no known contribution to iron metabolism, absorption, or storage [9]. Iron deposition within the gastric mucosa is known as gastric siderosis [10]. Deposition of iron in gastric mucosa is commonly seen in those with hemochromatosis, previous blood transfusions, hepatic cirrhosis, or excessive oral iron consumption.9 However, the precise mechanism by which this occurs is not well understood [10]. Moreover, diagnosis is established by identifying deposits endoscopically and confirmation made through histology. Grossly, endoscopic appearance of deposits appears as mucosal erosions, erythema, reactive gastropathy, and either yellow, brown, or even blue mucosal discolouration [9, 11].
On histology, the pattern of deposition is variable, and its recognition is useful for the management of patients. Three histological patterns of gastric siderosis have been described [10]. Pattern A refers to a ‘nonspecific’ distributive pattern whereby iron deposits are found within macrophages, stromal or epithelial cells. This pattern is the most common and is associated with gastric inflammation, ulcerations, or previous hemorrhage. Pattern B, known as ‘iron-pill gastritis’, is seen exclusively in those taking oral iron therapy with inorganic iron compounds, whereby the corrosive effects of excess iron intake directly on mucosal cells is seen. On histology, there are large extracellular clumps of crystalline iron depositing within stromal and epithelial cells resulting in mild gastritis. Pattern C, known as gastric glandular siderosis, demonstrates diffuse infiltrative iron deposition within fundic and antral glandular cells. It is found in conditions of iron overload and postulated to be contributed by portal hypertension where porto-caval shunting exposes gastric cells to high concentrations of iron [10, 11].
Our patient’s biopsy from the gastric antrum demonstrated pattern C gastric glandular siderosis along with features of pattern B. What makes our case unique is the combination of histopathological crossover between two distinct patterns. Our patient had no history of portal hypertension, hemorrhage, hemochromatosis, or previous blood transfusions. Baseline laboratory investigations including iron studies were normal and she was only on oral iron for a short time. This raises the question of the etiology behind the histological findings in our case. Marginean et al found 2 out of 3 patients with pattern C histology were not found to have any cause for their glandular siderosis [10]. We hypothesise our patient may have had glandular siderosis of unexplained etiology and super-imposed iron-pill gastritis from oral tablets of ferrous sulphate.
Little is known of the direct impact of iron deposition within gastric mucosa, and thus, poses several questions and clinical implications. Its recognition is important to allow clinicians to investigate further into the underlying cause. This case also raises awareness of the potential complications of inorganic oral iron supplements on the gastrointestinal system and for the need to regularly evaluate their necessity.
Authorship:
Authors DS, IM and TM were directly responsible for the patient’s management as well as the analysis, drafting, and approval of the submission.
Article Guarantor:
Deloshaan Subhaharan
Conflicts of Interest:
Nil
Sources of Funding:
Nil
Informed Consent:
Informed verbal consent was obtained from the patient for publication of this report and any accompanying images.
- Siah CW., Ombiga J., Adams LA (2006) Normal iron metabolism and the pathophysiology of iron overload disorders. Clin Biochem Rev 27: 5-16. [Crossref]
- Schrier SL., Auerbach M (2018) Treatment of iron deficiency anaemia in adults. UpToDate.
- Ewing D., Brozovich A., Bruns E (2019) Gastric siderosis and ulceration from intravenous iron supplementation manifesting as chronic upper gastrointestinal bleeding: a case report and review of the literature. Case Rep Gastrointest Med 2019: 1790686. [Crossref]
- Abbaspour N., Hurrell R., Kelishadi R (2014) Review on iron and its importance for human health. J Res Med Sci 19: 164-74. [Crossref]
- Yiannikourides A., Latunde-Dada G (2019) A short review of iron metabolism and pathophysiology of iron disorders. Medicines 6: 85. [Crossref]
- Wang J., Pantopoulos K (2011) Regulation of cellular iron metabolism. Biochem J 434: 365-381. [Crossref]
- Camaschella C., Nai A., Silvestri L (2020) Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 105: 260-272. [Crossref]
- Kohgo Y., Ikuta K., Ohtake T (2008) Body iron metabolism and pathophysiology of iron overload. Int J Hematol 88: 7-15. [Crossref]
- Kothadia JP., Arju R., Kaminski M (2016) Gastric siderosis: an under-recognized and rare clinical entity. SAGE Open Med 4: 2050312116632109. [Crossref]
- Marginean EC., Bennick M., Cyczk J (2006) Gastric siderosis: patterns and significance. Am J Surg Pathol 30: 514-520. [Crossref]
- De Petris G., Caldero SG., Chen L (2014) Histopathological changes in the gastrointestinal tract due to drugs. Int J Surg Pathol 22: 120-128.


