Abstract: Fibroblast growth factor receptors (FGFRs) are aberrantly activated in less than 10 percent of solid tumors, through different mechanisms such as single nucleotide variants, gene fusions and copy number alterations. In some types of cancer, such as urothelial or cholangiocarcinomas this frequency increases to 10–30%. Also, we see increased copy number alterations in breast cancer specifically after using cytotoxic therapies. There have been significant efforts to develop anti FGFR drugs. The landscape of anti-FGFR therapies does not include pan FGFR inhibitors as such there are only few drugs being assessed in early phase and randomized controlled clinical trials, such as erdafitinib and pemigatinib, and are approved by the Food and Drug Administration for the treatment of FGFR3-mutated urothelial carcinoma and FGFR2-fusion cholangiocarcinoma, respectively. Also, despite initial sensitivity to FGFR inhibition, acquired drug resistance leading to cancer progression develops in most patients.
Here we for the first time report a case series of patients with advanced cancer treated with multi targeted epigenetic therapy and we track their response on mutate allele frequencies of circulating DNA reflecting a positive and significant response in FGFR alterations. More importantly none of the cases reported developed resistance to anti FGFR approach. We conclude that such approach could change the standard practice of oncology specifically in the area of precision oncology and should be considered when FGFR alterations are considered drivers of tumor growth confirmed by liquid biopsy.
Fibroblast Growth Factors; Multi Targeted Epigenetic Therapy (MTET); Liquid Biopsy; Circulating Tumor DNA (Ctdna)
Biological processes that drive cell growth are exciting targets for cancer therapy. The fibroblast growth factor (FGF) signaling network plays a ubiquitous role in normal cell growth, survival, differentiation, and angiogenesis, but has also been implicated in tumor development [1-4]. Also, it has been shown that as other growth factors do, FGFR is significantly prognostic with poor prognosis seen in breast cancer, as an example [5-8].
Using technologies to detect circulating DNA (cDNA) in blood, has given us enormous ability to not only detect but also track and monitor responses longitudinally, as such there is an emerging need for clinical-grade molecular diagnostic tests to accurately detect these aberrations (FGRF) in both tumor tissues and blood samples [9-11]. This is essentially important when patients are at advanced stages and specifically after exhausting and failing standard therapies. For example, many times the molecular profiling of initial tumor may have not shown the alterations in FGFR, but after exposure to different therapeutics and because of tumor evolution, the metastatic disease manifests these alterations. The mechanism of such evolution is dependent on activation of stem cells, Notch1, Wnt and Snail and Slug, most of which are controlled by the microenvironment. One of the recent investigations looking at Pancreatic cancer and its tumor cell dissemination showed the strong collaboration of fibroblasts to promote the tumor cell access to the vasculture. There are also studies suggesting that KRAS activation only drives the tumor growth with cross talk through fibroblasts and their growth factors.
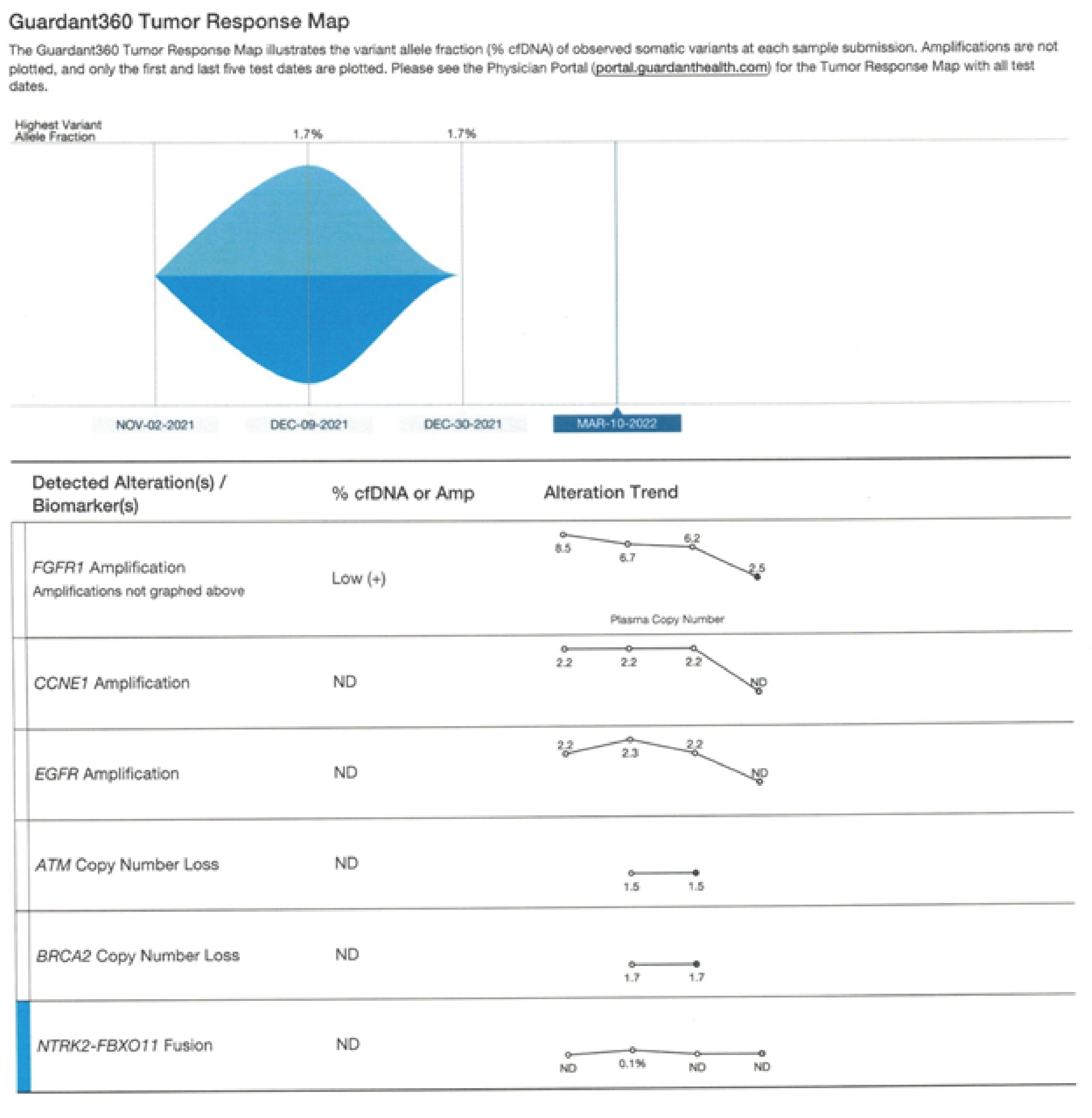
Figure 1:
Figure 2:
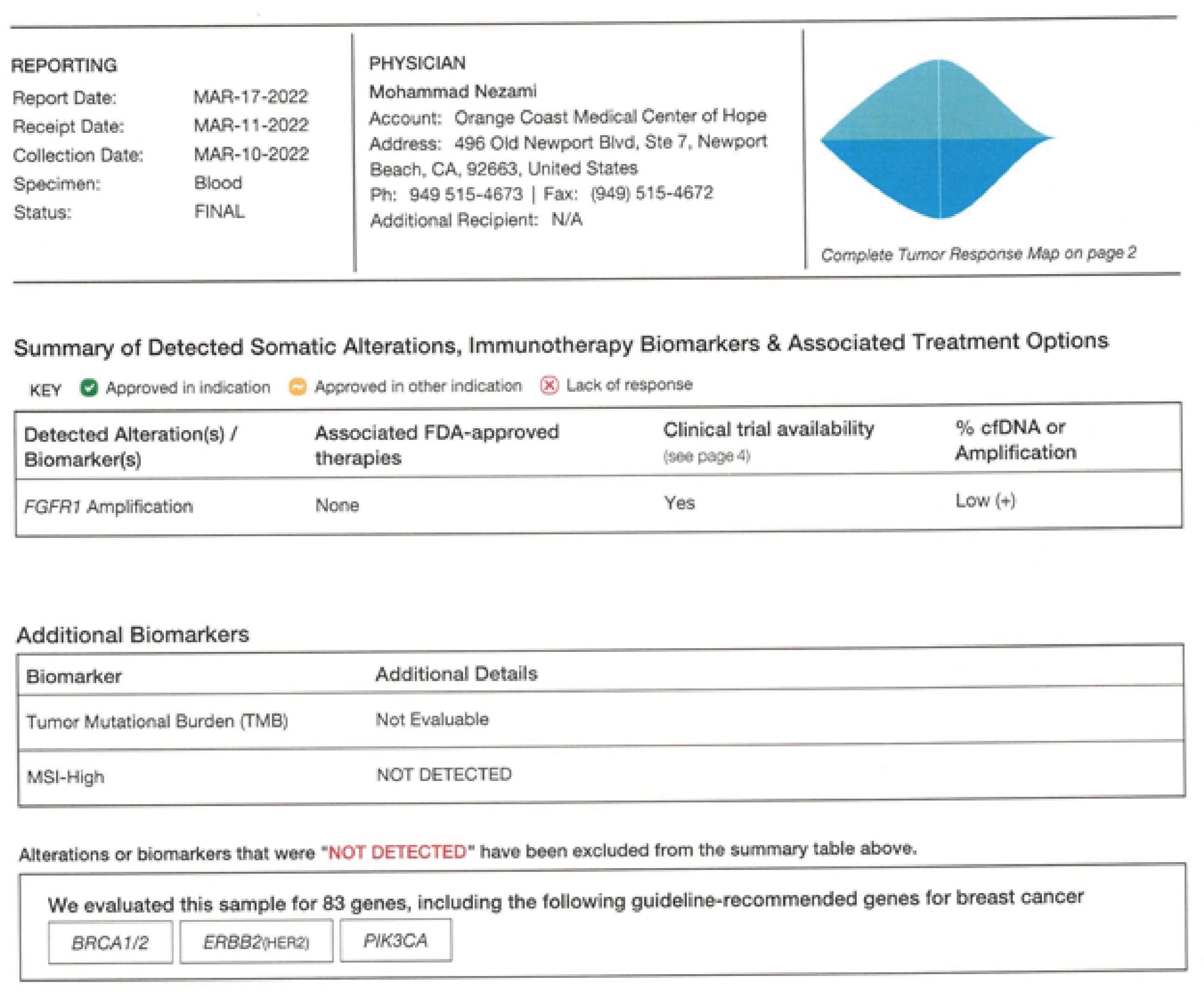
Figure 3:
Figurers 1-3. Guardant 360 reports a comparison of Pre and Post therapy- Complete resolution of CCNE1 and EGFR and reduction of FGFR1 mutated allele frequencies to 2.5 percent (+1) This data is captured in 2022
It is also known that amplification of the gene is more frequently observed that it’s mutation, for example about 20 percent of non-small cell lung cancers carry FGFR1 amplified gene. (compared to only 4 percent mutated FGFR2) [12-16]. Different reports have different numbers for example for FGFR (1-3) there has been a reported 64.8% SNVs and 35.9% rearrangements. FGFR1 amplifications are common in multiple cancer types, including hormone-receptor positive (HR+), human epidermal growth factor receptor 2-positive (HER2+), and triple-negative breast cancer patients at frequencies of 23%, 27%, and 7%, respectively. Fusion of the FGFR has also been detected in a variety of cancers, including breast cancer, urothelial carcinoma, glioblastoma, head and neck squamous cell carcinoma, low-grade glioma, lung adenocarcinoma, lung squamous cell carcinoma, ovarian cancer, prostate adenocarcinoma and thyroid carcinoma.
A validated commercial ctDNA assay (Guardant360) by Guardant laboratories have been used in studies looking at this alteration, specifically and results already published. In comparison when it was applied to 14 patients, which harboured 20 unique FGFR2/3 alterations, the assay was able to detect 4 out of 5 SNVs, 1 out of 2 amplifications and only 5 out of 13 fusions. Guardant 360 is also being used for the identification of resistance mutations in patients being treated with FGFR inhibitors, therefore we decided to use this test to detect and track both response and resistance to therapy in our case series.
The current landscape of FGFR inhibitors includes small-molecule receptor TKIs (non-selective, selective and covalent), monoclonal antibodies, FGF ligand traps and DNA/RNA aptamers. Many of these drugs are still under development. There are also combination protocols using these drugs with standard antineoplastic agents, such as chemotherapies. (pemigatinib combined with gemcitabine/cisplatin, pembrolizumab, docetaxel or trastuzumab (NCT02393248), and rogaratinib combined with atezolizumab (NCT03473756) [17].
Commercial ctDNA assay (Guardant360) were provided through Guardant laboratories. Patients had been informed, consented and treated with Multi Targeted Epigenetic therapies as off label use. The protocol consisted natural demethylators and histone deacetylase inhibitors (polyphenols). Between 2018 and 2022, we studied a total of 300 patients with solid tumor referred to our clinic for management, at advanced stages. The circulating DNA was measured in all available patients during this time, as we did not have access to this technology before 2018. The detection of cDNA was correlated with patient’s outcome through a longitudinal study after being treated. The tracking was only available for patients who received therapies and came for second blood draw to be retested. All patients were treated with multi targeted epigenetic therapies on daily basis per protocol until retested. The retest was performed at least 14 days after the initial testing. Patients did not change their diet or received any additional therapies during this time.
The target of interest was defined as Fibroblast Growth factor receptor (FGFR) amplification. There was a total of 28 cases identified, (7 male and 21 females, ages from 33 to 76) from which 18 cases were tracked and 6 did not desire to be treated and 4 although received therapies, could not be retested. The total responders to the therapy were 14 and non-responders were 4. The response range was between 0.1 mutated allele frequencies to 3.7. Average response was at 2.6. Duration of response was tracked up to 12 months at the time of this article (and continues in some of these subjects). The range of non-responders increased MAF was not more than 0.2. (3 patients with 0.1 and one patient with 0.2 percent). The response was statistically significant with average of (++) reduction of amplified gene expression manifested by direct inhibition of the FGFR. Tests for statistical significance were completed using the Fisher Exact probability test calculator found at vassarstats.net/tab2x2.html. Significance level was set at α = .05. Results: There was statistically significant (p-value: .046) correlation on the response of the allele frequency of the observed FGFR alteration with similar response in other biomarkers.
One of the very common side effects of all FGFR inhibitors is hyperphosphataemia, and we did not observe such effect in our patients clinically. Also, we did not observe any sign of resistance. Generally, resistance mechanisms involve the alternative pathway activation or mutations occurring at gatekeeper residues in FGFR, such as FGFR1 V561M and FGFR2 V565I, lead to steric hindrance within the ATP-binding pocket, which precludes the entry and binding of multiple FGFR inhibitor. One theory is that the epigenetic therapies used to treat the cases also targets the alternative pathways including PI3KCA and MAPK.
40 years old female with history of invasive ductal carcinoma ER/PR ++ diagnosed in 2016 status post mastectomy, refused conventional therapies altogether including hormonal therapies, chemotherapy and radiation, status post recurrence of her disease in stage four metastasized to the chest wall, ribs, and both lungs, with impaired breathing and mobility of her chest due to large sternal mass, numerous bilateral pulmonary lesions and skeletal mets referred to us from Texas for evaluation and treatment. After her criteria was reviewed, informed consents were obtained, she was enrolled in a phase II clinical trial at our clinic using a nano particled polyphenol (NP-QT) which she received on daily basis, four times a week for four weeks, at total dose of 1000mg/m2 per week intravenously.
Her initial findings confirmed a germline mutation at SMAR, SNF/SWI. Her liquid biopsy was obtained before she started the trial as well as a whole-body staging PET/ CT, quality of life measures was obtained through EORTC and labs including tumor markers and growth factors as well as circulating tumor cell analysis ordered. Her labs indicated elevated CEA, positive and very high amplifications at her liquid biopsy for several alterations (FGRF, CCNE1 and EGFR), but her CTC was negative (Biofocus Lab).
Immediately after starting her on the treatment, she reported that the pain had started to subside in her chest wall, and the mass was less pressing to the sternum. Her QOL has improved post treatments by EROTC indices (Quality of Life for Cancer Patients questionnaire). In exam the palpable mass in sterum shrunk 50 percent after 4 weeks. She was restaged with a PET scan which confirmed stable to improved findings after 4 weeks of therapy. Intensively active right sternal mass with the huge size of 13.8X 8.7 cm and metabolic activity SUV of 8.0 was stable and had less metabolic activity. Right axillary, internal mammilary, and hilar lymhadenopathies remained stable and (10/27/21 and 12/15/2021). 9th rib lesion and pleural metastasis were diminished.
Her c DNA reported reduction of FGFR from 8.5 to 3.5 and other alterations (EGFR and CCNE1 became non detectable, after 15 days of the trial. (measured on 11/29/2021).
Further her FGFR1 dropped down to 2.5 on 3/11/22, as she continued the care with maintenance IV therapies at once a week schedule.
On March 10th,2022 she was reevaluated and her Guardant showed complete resolution of CCNE1 and EGFR and reduction of her FGFR1 down to 2.5 (please see Figures 1,2,3).
She continues to improve with the therapies and significant response manifested in all her markers. This data is captured in 2022.
We essentially believe that research addressing growth factors in general and fibroblast growth factors specifically will lead to a deeper understanding of cancer biology that can subsequently be exploited to improve patients care and outcomes. Inhibition of FGFR alterations using a different approach with minimal side effects and no resistance is desirable and ultimately superior to current available technologies. We initially presented 374 case studies treated with this technology in American Society of Clinical Oncology (ASCO) 2019 and published the data in Journal of Clinical Oncology (JCO) same year. The targets of interest included growth factors. Here we present a case series with advanced solid tumors for which we had designed specific targeted therapies and tracked their response, monitored over years.
To our knowledge this is the first report on longitudinal monitoring of FGFR alteration with liquid biopsy in response to Epigenetic therapies. As our knowledge evolves about growth factors, we appreciate their role in clinical outcome and the importance of drug resistance through microenvironmental cross talk. This could open the door for further research and generating hypothesis to address unanswered questions about tumor stroma relation and further enhance our ability to provide more meaningful clinical responses at clinic.
Our institution does not require ethical approval for reporting individual cases or case series. Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Kelly Adams MS, Guardant Health, 505 Penobscot Dr, Redwood City, CA 94063
No conflict of interest is claimed by authors
- Babina, I. S., Turner, N. C (2017) Advances and challenges in targeting FGFR signaling in cancer. Nat. Rev. Cancer 17: 318-332. [Crossref]
- Ornitz, D. M., Itoh, N (2015) The fibroblast growth factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol 4: 215-266. [Crossref]
- Helsten, T., Elkin, S., Arthur, E., Tomson, B. N., et al. (2016) The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin. Cancer Res 22: 259-267. [Crossref]
- Imamura, T (2014) Physiological functions and underlying mechanisms of fibroblast growth factor (FGF) family members: recent findings and implications for them pharmacological application. Biol. Pharm. Bull 37: 1081-1089. [Crossref]
- Sobhani, N., Ianza, A., D’Angelo, A., Roviello, G., Giudici, F., et al. (2018) Current status of fibroblast growth factor receptor targeted therapies in breast cancer. Cells 7: 76. [Crossref]
- André, F., Cortés, J (2015) Rationale for targeting fibroblast growth factor receptor signaling in breast cancer. Breast Cancer Res. Treat 150: 1-8. [Crossref]
- Roidl, A., Foo, P., Wong, W., Mann, C., Bechtold, S., et al. (2010) The FGFR4 Y367C mutant is a dominant oncogene in MDA-MB453 breast cancer cells. Oncogene 29: 1543-1552. [Crossref]
- Ray, M. E., Yang, Z. Q., Albertson, D., Kleer, C. G., Washburn, J. G., et al. (2004) Genomic and expression analysis of the 8p11–12 amplicon in human breast cancer cell lines. Cancer Res 64: 40-47. [Crossref]
- Benayed, R., Offin, M., Mullaney, K., Sukhadia, P., Rios, K., et al. (2019) High yield of RNA sequencing for targetable kinase fusions in lung adenocarcinomas with no mitogenic driver alteration detected by DNA sequencing and low tumor mutation burden. Clin. Cancer Res 25: 4712-4722. [Crossref]
- Mouliere, F., Chandrananda, D., Piskorz, A. M., Moore, E. K., Morris, J., et al. (2018) Enhanced detection of circulating tumor DNA by fragment size analysis. Sci. Transl. Med 10: 4921. [Crossref]
- Moss, T. J., Ahnert, J. R., Oakley, H. D., Kahle, M., Karp, D. D., et al. (2019) Baseline cfDNA characteristics and evolution of cfDNA profile during treatment with selective FGFR inhibitor TAS-120. J. Clin. Oncol 37: 3056.
- Paik, P. K., Rudin, C. M., (2016) Missing the mark in FGFR1-amplified squamous cell cancer of the lung. Cancer 122: 2938–2940. [Crossref]
- Ng, T. L., Yu, H., Smith, D. E., Boyle, T. A., York, E. R., et al. (2019) Preselection of lung cancer cases using FGFR1 mRNA and gene copy number for treatment with ponatinib. Clin. Lung Cancer 20: e39-e51. [Crossref]
- Hibi, M., Kaneda, H., Tanizaki, J., Sakai, K., Togashi, Y., et al. (2016) FGFR gene alterations in lung squamous cell carcinoma are potential targets for the multikinase inhibitor nintedanib. Cancer Sci 107: 1667–1676. [Crossref]
- Fumarola, C., Bozza, N., Castelli, R., Ferlenghi, F., Marseglia, G., et al. (2019) Expanding the Arsenal of FGFR Inhibitors: A Novel Chloroacetamide Derivative as a New Irreversible Agent With Anti-proliferative Activity Against FGFR1-Amplified Lung Cancer Cell Lines. Front. Oncol 9: 179. [Crossref]
- Morgensztern, D., Karaseva, N., Felip, E., Delgado, I., Burdaeva, O., et al. (2019) An open-label phase IB study to evaluate GSK3052230 in combination with paclitaxel and carboplatin, or docetaxel, in FGFR1-amplified non-small cell lung cancer. Lung Cancer 136: 74-79. [Crossref]
- Schuler, M., Cho, B. C., Sayehli, C. M., Navarro, A., Soo, R. A., et al. (2019) Rogaratinib in patients with advanced cancers selected by FGFR mRNA expression: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol 20: 1454-1466. [Crossref]



