All nuclei of elements consist of successive chain of nuclei of helium. Stability of nuclei of helium is provided by an exchange by mesons between nucleons. Division of uranium 235U takes place in a chain where value of energy of separation of nucleus of helium 4He close to the zero.
Successive chain of nuclei of helium or isotopes of nuclei of helium stability is provided by cooperation of nuclides.
It is known that the number of nucleons in a nuclei is multiple four plays a large role at determination of properties of nucleus Foremost at the nuclei of containing an even number protons and neutrons spin of nucleus equal to the zero. To this group of elements belong helium 4He,carbon 12C,oxygen 16O.Beecause the nucleus of helium 4 He is the simples ,then it serves as basis for the construction of all anther nuclei. .
Substantive Provisions
Basis (by a brick) for formation of nuclei of elements is a nucleus of helium 4He or isotope nucleus of helium. All other nuclei of elements consist of successive chain of nuclei of helium. Why did the nucleus of helium become basis all other nuclei?
Appearance of fourth sector (proton or neutron) in the chart of tritium (3H) or chart of isotope of helium (3He) creates the complete structure of nucleus of helium. Thus sharply the size of binding energy grows to 28.296 MeV and binding energy on nuclide to 7.04 MeV. And no another way thus to increase the value of binding energy exists. Therefore the nucleus of helium becomes basis for all elements.
The nucleus of helium consists of two protons and two neutrons. A proton is a stable particle and neutron is unstable. How to provide stability of nucleus of helium? Stability of nucleus is provided by cooperation of protons and neutrons by means of π-mesons. We will consider one of variants of such cooperation. (Figure 1)
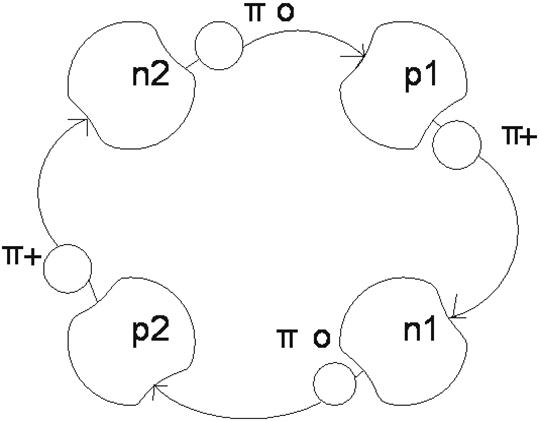
Figure 1.
How do nucleus cooperate in a nucleus? They are constrained and cooperate all together. Protons and neutrons are difficult systems consisting of central part and cloud of the continuously emitted and taken in mesons. The proton P1 emits a π+meson and he is taken in by the neutron n1.The proton p2 emits the same π+meson, that is taken in by the neutron n2. And at same time neutrons emit π-0 mesons. That is taken in by protons .After an exchange of the mesons proton and neutron change by rotes. A proton becomes a neutron and former neutron by a proton. This cooperation recurs in the next loop. A current flows in the ring of helium, because the charge of 1e moves, that creates the magnetic field. This magnetic field helps the orientation of nucleus of helium in relation to other nuclei.
The nuclei of elements consist of chain of nuclei of helium that is bound by inter se binding energy of equal energy of separation of nucleus of helium. This connection come a true by a transmission between nuclei of part of energy. This cooperation connection ends with in nuclei. Why does this chain coagulate in a ball? Maybe she aims to occupy a minimum volume in space.
Why do the nuclei of all elements consist of a sequential chain of helium nuclei? We start by analyzing the helium core. We divide the helium core into two equal parts. To find the energy of separation of a part of the nucleus from the whole nucleus it is necessary to subtract from the binding energy of the whole nucleus the binding energy of its two parts. BE-binding energy, ES-energy of separation. ES (2H) (4He) =BE(4He)-BE(2H)-(BE2H)=28.296-2.225-2.225=23.846MeV.
Next, we will study the 52Fe core .To analyze the 52Fe core we will use the same principle as for the helium core. Although this method is applicable with restrictions for the 52Fe the following factors are not taken into sccount: 1) the shape of the nucleus 2) the influence of a positive charge. Divide the 52FE core into two equal parts.
1). ES (26Al) (52Fe)=BE(52Fe)-BE(26AL)-BE(26Al)=447.705-211.892-211.892=23.921MeV
We obtained a value almost equal to the value of the division of the helium nucleus into two parts We assume that the helium nucleus is located in the center of the 52Fe nucleus. (Figure 2)
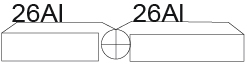
Figure 2.
2).Next, we divide the 52Fe core into unequal parts. One part is 26Na and the second part is 30P.
ES (22Na) (52Fe)=BE(52Fe)-BE(22Na)-BE(30P)=447.705-174.148-250.409=23.148
Again, a value comparable to the fission of the helium nucleus was obtained. In this case we have a division into two parts 22Na and 30P.One fission point is located to the left of the center of the nucleus and the second to the right of the center Thus , we already have three parts of the 52Fe nucleus consisting of helium nuclei.. (Figure 3)
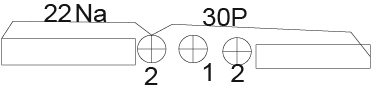
Figure 3.
3). Now we divide the 52Fe core into two parts consisting of 18F and 34Cl.
ES (18F) (52Fe)=BE(52Fe)-BE(18F)-BE(34CL)=447.705-137372-285.568=24.765MeV (Figure 4)
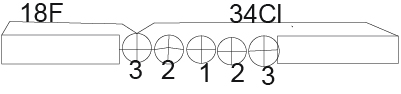
Figure 4.
Now already have five parts of the 52Fe nucleus.
4). Let us do four more calculations similar to those done above.
ES (14N)(52Fe)=BE9(52Fe)-BE(14N)-BE(38K)=447.705-104.661-320.650=22.394MeV (Figure 5)
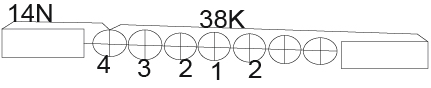
Figure 5.
5). ES (10B) (52Fe)=BE(52Fe)-BE(10B)-BE(42Sc)=447.705-64.753-354.692=28.260MeV
6).ES (6Li) (52Fe)=BE(52Fe)-BE(6Li)-BE(46V)=447.705-31.995-390.365=25.345MeV
7).ES (2H) (52Fe)=BE(52Fe)-BE(2H)=BE(50Mn)=447.705-2.225-426.640=18.840MeV
Thus, we got all 13 helium nuclei that make up the 52Fe nucleus. (Figure 6)
BE (52Fe) =13х28.296+(-0.092)+7.367+7.162+4.725+9.322+9.985+6,947+6.641+7.050+5.125+7.696+7.937=447.705Me (Figure 6) (Table 1)
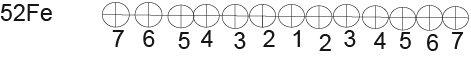
Figure 6.
Table 1. Formation of Nuclei of 235U and 238U
|
92 |
184 |
U |
220 |
221 |
222 |
223 |
224 |
225 |
226 |
227 |
228 |
229 |
230 |
231 |
232 |
233 |
234 |
235 |
|
|
238 |
|
90 |
180 |
|
|
|
|
|
|
|
|
|
|
|
|
|
229 |
|
|
232 |
5.710 |
|
88 |
176 |
|
|
|
|
|
|
|
|
|
|
|
223 |
|
|
226 |
|
4.538 |
|
|
86 |
172 |
|
|
|
|
|
|
|
|
|
217 |
|
|
220 |
|
8.501 |
|
|
|
|
84 |
168 |
|
|
|
|
|
|
|
211 |
|
|
214 |
|
|
6.253 |
|
|
|
|
|
82 |
164 |
|
|
|
|
|
205 |
|
|
208 |
|
|
10.998 |
|
|
|
|
|
|
|
80 |
160 |
|
|
|
199 |
|
|
202 |
|
|
12.650 |
|
|
|
|
|
|
|
54 |
|
78 |
156 |
|
|
193 |
|
|
196 |
|
|
10.636 |
|
|
|
48 |
49 |
50 |
51 |
52 |
53 |
|
|
76 |
152 |
187 |
|
|
190 |
|
|
10.591 |
|
|
|
|
|
|
|
|
187 |
|
|
190 |
|
74 |
148 |
35 |
36 |
37 |
38 |
39 |
40 |
41 |
42 |
43 |
44 |
45 |
46 |
47 |
|
181 |
|
|
184 |
|
|
|
72 |
144 |
|
|
|
|
|
|
|
|
|
|
|
|
|
176 |
|
178 |
|
9.180 |
|
|
70 |
140 |
|
|
|
|
|
|
|
|
|
|
|
171 |
172 |
|
|
5.770 |
|
|
|
68 |
136 |
|
|
|
|
|
|
|
|
|
|
|
166 |
167 |
|
6.500 |
|
|
|
|
|
66 |
132 |
|
|
|
|
|
|
|
|
|
161 |
162 |
|
|
9.454 |
35 |
36 |
37 |
38 |
|
64 |
128 |
|
|
|
|
|
|
156 |
157 |
|
|
|
9..359 |
|
|
|
|
|
62 |
124 |
|
|
|
|
150 |
151 |
152 |
|
|
|
|
5.036 |
|
|
|
|
|
60 |
120 |
|
|
144 |
|
146 |
147 |
|
|
|
|
|
5.006 |
|
|
|
|
|
58 |
116 |
|
|
140 |
141 |
142 |
|
|
|
|
|
|
8.606 |
|
|
|
|
|
56 |
112 |
|
|
136 |
137 |
|
|
|
|
|
|
|
16.763 |
|
|
|
|
|
54 |
108 |
|
|
|
131 |
|
|
|
|
|
|
|
|
|
16.792 |
|
|
|
|
|
52 |
104 |
|
125 |
|
|
|
|
|
|
|
|
|
|
|
16.550 |
|
|
|
|
|
50 |
100 |
119 |
|
|
|
|
|
|
|
|
|
|
|
|
|
20.699 |
|
|
|
119 |
|
48 |
96 |
|
|
|
|
|
|
|
|
|
|
|
|
|
18.200 |
|
113 |
|
|
|
46 |
92 |
20 |
21 |
22 |
23 |
24 |
25 |
26 |
27 |
28 |
29 |
30 |
31 |
32 |
|
107 |
ES(a+2n) = 18.07 |
|
|
44 |
88 |
|
|
|
|
|
|
|
|
|
|
|
|
101 |
|
ES (a+n) =9.666 |
|
|
|
42 |
84 |
|
|
|
|
|
|
|
|
|
|
|
96 |
|
2.761 |
|
|
|
|
|
40 |
80 |
|
|
|
|
|
|
|
|
|
|
|
|
92 |
|
2.198 |
|
|
|
|
|
38 |
76 |
|
|
|
|
|
|
|
|
|
|
|
|
88 |
|
7.916 |
|
|
|
|
|
36 |
72 |
|
|
|
|
|
|
|
|
|
|
|
|
84 |
|
ES(α+n) =17.904 |
|
|
|
|
34 |
68 |
|
|
|
|
|
|
|
|
|
|
79 |
|
22.220 |
|
|
|
|
|
|
32 |
64 |
|
|
|
|
|
|
|
|
73 |
|
21.013 |
|
|
|
|
|
|
|
|
30 |
50 |
|
|
|
|
|
|
67 |
|
ES(α+2n) =21.254 |
|
|
|
|
|
|
|
|
28 |
56 |
|
|
|
|
|
61 |
|
ES(α+n) =15.005 |
|
|
|
26 |
52 |
|
|
|
56 |
|
|
7.614 |
|
|
|
|
|
|
|
|
|
|
|
|
24 |
48 |
|
|
|
|
52 |
|
|
7.696 |
|
|
|
|
|
|
|
|
|
|
|
|
22 |
44 |
|
|
|
|
48 |
|
|
5.125 |
|
|
|
|
|
|
|
|
|
|
|
20 40 |
|
|
|
|
44 |
|
ES(α)=7.050 |
|
|
|
|
|
|
|
|
|
|
|
|
18 |
36 |
|
|
|
|
40 |
|
ES(a+2n) |
=26.033 |
|
|
|
|
|
|
|
|
|
|
|
16 |
32 |
|
|
34 |
|
|
|
ES(α+2n) =15.302 |
|
|
|
|
|
|
|
|
|
|
|
14 |
28 |
28 |
|
|
9.985 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12 |
24 |
24 |
|
|
9.322 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10 |
20 |
20 |
|
|
4.730 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8 |
16 |
16 |
|
|
7.162 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
6 |
12 |
12 |
|
|
7.367 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4 |
8 |
8 |
|
ES(α)=0.092 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Z |
2Z |
n |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
19 |
Why are the 235U uranium nucleus divided and the 238U nucleus not divided? Where is the fission point? The uranium nucleus consists of 46 helium nuclei or helium nuclei isotopes. They are interconnected by the binding energy which we traditionally call the separation energy ES(a) or ES(a+n)or ES(a+2n).The magnitude of this energy depends on the following factors:1)the magnitude of Z 2)the type of helium nucleus 3) the magnitude of the Coulomb energy. (Figure 7) (Table 2 and 3 table)
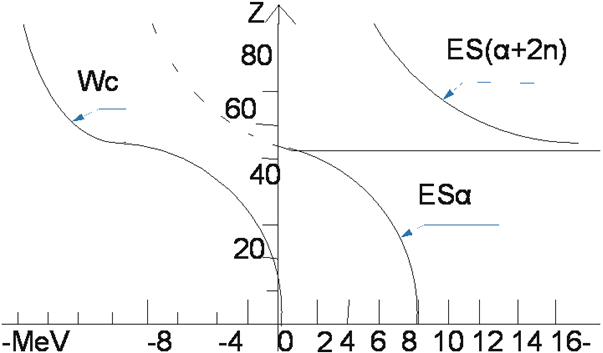
Figure 7.
Table 2.
64 ES (a+2n) =13.080 |
|
|
|
|
|
156 |
157 |
ES (a+1n) =7.403MeV |
62 ES (a+2n) =10.900 |
|
|
|
150 |
|
152 |
|
ES (a+1n) =8.003 |
60 ES (a) = -1.012 |
|
144 |
|
|
147 |
|
|
ES (a+1n) =5.006 |
58 ES (a) =1.629b |
|
140 |
|
142 |
|
|
|
ES (a+1n) =8.606 |
56 ES (a+n) =11.401 |
|
136 |
137 |
|
|
|
|
ES (a+2n) =16.388MeV |
54 |
131 |
|
|
|
|
|
|
|
Table 3.
64 ES (a+n) = 9.366 |
|
|
|
|
156 |
167 |
ES (a+1n) =7.403MeV |
62 ES (a+n) =5.003 |
|
|
|
151 |
152 |
|
ES (a+1n) =8.003 |
60 ES (a+n) =6.880 |
|
|
146 |
147 |
|
|
ES (a+1n) =5.008 |
58 ES (a) =0.144 |
|
141 |
142 |
|
|
|
ES (a+1n) =8.206 |
56 ES (a+2n) =16.388 |
|
137 |
|
|
|
|
ES (a+2n) =16.388MeV |
54 |
131 |
|
|
|
|
|
|
Table1 shows the formation scheme of the 235U and 238U isotopes. It is known that the 235U isotope is divided into two parts and the 238U isotope is not divided. What is the difference?
Let us consider in more detail. Tabl.2 (part of Tabl1) shows the path to the sequential formation of the a uranium element from Z54 to Z64.Prior to A131 the formation of the 235U and 238U isotopes proceeds along the same branch. Starting with A131 the isotope paths diverge Isotope 235U goes along the branches A131 A136 A140 A14 ,A15 ,A156 and further along the left branch to the end.
At the A136 A140 transition the binding energy between helium nuclei becomes 1.629MeV and at theA140,A144 transition the binding energy becomes negative ES(a)=-1.012MeV. This is the transition which is a weak point and causes the division of the 235U isotopes. The formation of 238U isotope proceeds along the path A131,A137,A142,A147 A152,A157,A162,A167 and further along the right branch to A238. There are no net places in this branch.The second variant of the formation of these isotopes in Tabl.3.Prior to A137 the formation 0f 235U and 238U isotopes proceeds along the same branch. Beginning with A137 the isotope paths diverge. The 238U isotope follows the same path and the 235U isotope follows the A137 A141 A146 A151, A156 path and further along the previous branch to the end. There is a weak point in the transition between A137 and A141. The binding energy between the helium nuclei here is 0.144MeV .The second variant of the formation of the 235U nucleus is more likely. This is the place where the chain can be broken into two parts by a thermal nutron.
Dividing of uranium by two parts making 2/3 basic nucleus takes place the break of successive chain of nucleus where a value ES (a) approaches a zero.
- Buchakchiiskiy FF (2018) Model of Nuclear. J Phys Astron Rev 6 : 1. [crossref]
- Buchakchiiskiy FF (2019) About the model of nuclear. 4: 2.
- Global National Data Center 2017.
- Centre for Photonuclear Experiments 2017. [crossref]







