Background: Cytotoxicity tests are undertaken in the investigation of the biocompatibility of biomaterials used in human and veterinary medicine to evaluate the possibility of toxic substance release.
Aims of the Study: The aim of the present work was to evaluate the cytotoxicity of arch wire on human gingival cells in orthodontic patients under supportive phase of periodontal therapy.
Subjects and Methods: The sample of the present study consisted of 160 patients (40 patients per group).They were selected from the outpatient clinics of our training centers. All patients in group II, III and IV received orthodontic and periodontal therapy in different intervals(less than 6 months, between 6-12 months and more than 12 months). Plaque index (PI), and gingival index (GI were recorded. The cytotoxic effect of archwire was evaluated by Cytological investigation (MN Apoptotic and Necrotic Test). The results were collected and analyzed by NOVA test statistical analysis.
Results: In the present study there were significant differences were detected in all samples of all patients groups compared to patients control groups in plaque index (PI), and gingival index (GI), there was no significant induction of micronucleus, apoptosis and necrosis. Comparing the results showed that normal group (with no wire) had the lowest value of MN, while the MN frequency increased very slightly with non-significantly value in other three groups.
Conclusion: Our research showed significant variation of Micronucleus formulation between regions, this variation might due to the traditional nutrition ways but not to wire installation.
Cytotoxicity Tests: Gingival Cells: Orthodontic Archwires
Orthodontic treatment may initiate oral clinical manifestations, such as labial desquamation [1] multiform erythema [2], gingivitis [3], and gingival enlargement [4]. Such manifestations are usually associated with the inflammatory response induced by the corrosion of orthodontic appliances, and major emphasis has been placed on nickel [5]. Inflammatory response to nickel is considered as type IV hypersensitivity and is manifested as nickel allergic contact stomatitis but it is an etiology has not yet clearly been defined [6].
Throughout interceptive and corrective treatments, orthodontic bands are commonly used in daily practice. The bands are usually made of stainless steel and consist of nickel, iron and chromium, and are considered a biocompatible alloy [7]. However, in several clinical situations, it is necessary to connect orthodontic wires to the bands, especially when auxiliary appliances, such as lingual arches and maxillary expanders are made. Due to its proven performance low cost and ease of use, silver solder is the alloy of choice for connecting the support wires to the appliances. Nevertheless, the silver solder alloy comprises iron, copper and zinc. These ions present a major tendency to be released to the buccal cavity [8] and they may have cytotoxic effects, resulting in decrease of cell viability [9]. Cadmium used to be added to the composition of silver solder alloys some decades ago [10] and, due to the process of zinc obtaining from the ores, cadmium may appear as a zinc contaminant [11]. It is important to remember that cadmium exposure is responsible for hepatic, renal, and myocardial damage characterized by increased creatinine, total and direct bilirubin concentrations and increased ALT and lactate dehydrogenase (LDH) activities [12]. Besides this cadmium has been considered a mutagen and may be related to the occurrence of cancer [13-15]. Laser welding can be an alternative to soldering with silver solder. The energy generated by laser soldering promotes real fusion of the joined metals. It may be less susceptible to corrosion and thus more biocompatible.
The aim of the present work was to evaluatethe cytotoxicity of arch wire on human gingival cells in orthodontic patients under supportive phase of periodontal therapy.
Inclusion Criteria
Patients systemically healthy
Patients had orthodontic appliances according the duration that mention below
Exclusion Criteria
Smoker patient
Patient with prosthetics appliances
Pregnant women
Patients Sampling
One hundred and sixty patients systemically healthy were selected from the out patients clinics of our training centers in:
- Najran, Abha cities (south region in Saudi Arabia) 80/160 total of patients.
- Riyadh city (central region of Saudi Arabia) 80/160 total of patients.
Group I: Forty patients were control group.
Group II: Forty patients received orthodontic treatment for six months.
Group III: Forty patients received orthodontic treatment between six months to 12 months.
Group IV: Forty patients received orthodontic treatment for more than 12 months
Clinical examination
Plaque index (PI) and gingival index (GI) were recorded for all patients (Figure 1&2).
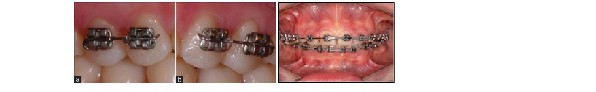
Figure 1.Photographs of brackets and write fixed with (a) metal and (b) elasticties

Figure 2.Clinical view
Cytological Investigation (MN Apoptotic and Necrotic Test)
Epithelial cells were collected from gingival were smeared on to clean microscope glass slides. The cells were fixed with cold 100% methanol. The slides were aged at37OC overnight and then stain with Giemsa, May-grunwald.1000 nucleated epithelial cells will analyze for the presence of MN Necrotic and apoptotic cells at a final 40x magnification for each participant. The cells harboring micronucleus necrotic and apoptotic had been recorded
Figure 3.microscopic images of cytology
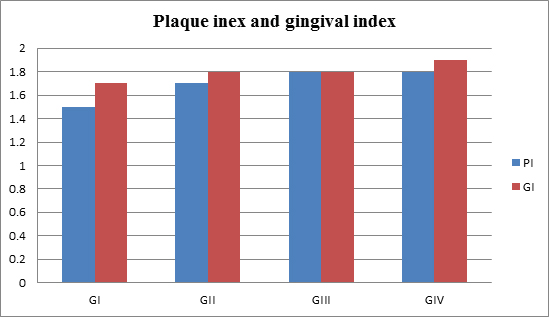
Figure 4.Sample on slide
Plaque index (PI), gingival index (GI) and Changing of MN incidence was calculated as percentage for each group and compared with control by One-Way ANOVA, and Student T-test. Statistical significance will determine.
Figure (5) summarized the mean and Mean and SD± of periodontal clinical parameters of all patients groups. There were significance differences in plaque index (PI), and gingival index (GI), compared to the patients in control group.
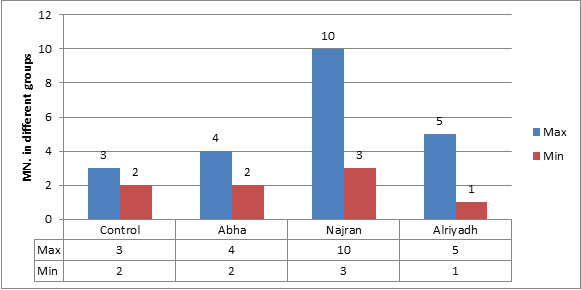
Figure 5.Clinical parameters
There was no significant induction of micronucleus, apoptosis and necrosis. Comparing the results showed that normal group (with no wire) had the lowest value of MN, while the MN frequency increased very slightly with non-significantly value in other three groups (Figure 6). Whereas results showed a significant increase between regions, Najran has the max average (Figure 7). No significant related to the periods of installation (table 4).The microscopic investigation of Micronuclei also showed a variation in their shapes and number per cell as show. The micronucleus type (M1) was found in all groups, while, type (M2) and (M3) micronucleus were not found in any group (Figure 8). Where the deformed nucleus (irregular in shape and has loops) and necrotic cells were not detected in any group.
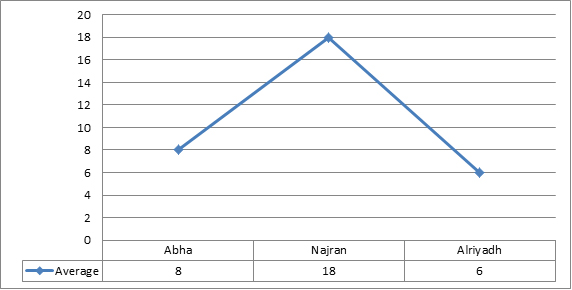
Figure 6.Average of maximum and minimum value of MN. Apoptotic and necrotic exfoliated cells in all groups
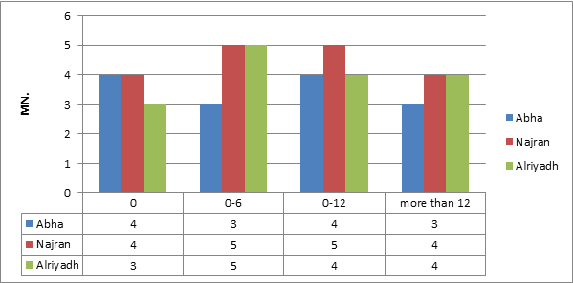
Figure 7.Average of MN. Comparison table
The aim of the present work was to evaluatethe cytotoxicity of arch wire on human gingival cells in orthodontic patients under supportive phase of periodontal therapy. Cytotoxicity tests are undertaken in the investigation of the biocompatibility of biomaterials used in human and veterinary medicine to evaluate the possibility of toxic substance release. The selection of the type of cells for cytotoxicity assessments is dependent on the type of tissue the biomaterial is in contact with under in vivo conditions.
Cytotoxicity tests on the osteoblasts (OBs) and fibroblasts are carried out to evaluate the biocompatibility of orthodontic implants or orthodontic appliances [16]. There is a need to elaborate on an experimental methodology of cytocompatibility testing of the materials used in medicine: the applied concentration of metal ions and the type of exposure, and culturing cells directly on the material or in media containing the products of its corrosion. In the case of implanted alloys, such as dental or hip implants, culturing on the surface seems to be more rational. However, cell cultivation in model media with a precisely estimated concentration of metal ions in preliminary experiments, e.g., in artificial saliva/biomaterial, seems more appropriate in cytotoxicity tests for dental materials. This is probably a more suitable method for the evaluation of cytotoxic effects of the materials of which orthodontic fixed appliances are made.
Plaque is the major etiologic factor in the development of gingivitis [17]. Experimental animal studies have shown that in the absence of plaque, orthodontic forces and tooth movements do not induce gingivitis. In the presence of plaque, however, similar forces are capable of inducing angular bone defects and with tipping and intruding orthodontic tooth movements, attachment loss can occur [18]. In healthy, reduced periodontal tissue support regions, orthodontic forces kept within the biological limits do not cause gingival inflammation [19]. The most important factor in the initiation, progression and recurrence of periodontal disease in reduced periodontium is the presence of microbial plaque [20]. Following placement of a fixed appliance; some amount of gingival inflammation is noticeable in most patients that is usually transient and does not lead to attachment loss [21]. Gingival hyperplasia can be a potential problem around orthodontic bands, leading to pseudo-pocketing. However, this usually resolves within weeks of depending. The importance of plaque control and good oral hygiene must be stressed to the patient before starting the fixed appliance treatment and adequate patient compliance must be ensured throughout treatment to prevent gingival inflammation. In the present study there was increased in plaque index (PI) and gingival index (GI) in all patients group compared to the patients of control group. Orthodontic materials have the potential to induce allergic reactions in some patients [22]. Nickel present in orthodontic appliances like brackets, bands, and archwires is responsible for causing allergic reactions in some patients.
Latex found in gloves, elastics, and elastomeric ligatures may cause reactions to the protein antigen present in rubber in a few patients. Methyl methacrylate found in bonding agents and composite is also responsible for allergic reactions in rare cases [23].
Biocompatibility depend on the ability of a biomaterial to perform its ideal function with regard to a medical therapy, without causing any adverse local or systemic effects in the recipient or benefit of that therapy but producing the most suitable effective cellular or tissue response in that particular situation and maximizing the clinically relevant effectiveness of that therapy [24].
While evaluating the biocompatibility of orthodontic metal components, corrosion is the main concern. The release of several metallic ions [25] may lead to hypersensitivity and allergic reactions, either locally as well as systemically [26]. The orthodontic metal wire has progressed from nickel-chromium-iron (Ni-Cr-Fe) stainless steel (SS) alloy to nickel-titanium (NiTi) alloy. This is related to its properties: (1) super elasticity, (2) thermal shape memory, (3) good corrosion resistance, and (4) good biocompatibility.
Different metal alloys have a wide range of applications in clinical orthodontic treatment. NiTi wire alloy's characteristics can reduce chair time and shorten treatment times [27].
Brackets used in orthodontic treatment include the mechanisms by which the arch wire can shift force to the teeth. The orthodontic brackets may be Biodegraded in the warm and moist conditions of the mouth. Moreover, it is an effective way to release metal ions that can cause side effects on oral mucosa and gingiva or alveolar bone.
Clinically, each orthodontist should concentrate on using low cytotoxic materials to reduce the negative impact. Schuster et al. [28] recommended that based on careful attention to patients’ case histories, the orthodontist should use ceramic, plastic or titanium brackets for fixed appliance therapy in patients known to have a nickel allergy. However, it is a matter of dispute whether plastic brackets should be used, because of the potential action of various polymers at subtoxic levels [29] .Pereira et al. [30] the effects of 2 metal and 2 ceramic brackets on the buccal mucosa epithelial cells in 21 individuals of both sexes were investigated. With the use of liquid-based exfoliative cytology, morphometric and morphologic changes in buccal mucosa cells adjacent to these brackets were determined. They concluded that buccal mucosa cells attached to the metal brackets demonstrated significant changes than any of those attached to the ceramic brackets, and buccal mucosa cells attached to the metal and ceramic brackets seemed to return to their original morphology after the brackets were removed [31,32].
Our research showed significant variation of Micronucleus formulation between regions, this variation might due to the traditional nutrition ways but not to wire installation.
- Lindsten R, Kurol J (1997) Orthodontic appliances in relation to nickel hypersensitivity: a review. Journal of Orofacial Orthopedics 58(2): 100–108. [Crossref]
- Starkjaer L, Menne T (1990) Nickel allergy and orthodontic treatment. European Journal of Orthodontics 12(3): 284–289. [Crossref]
- Shelley BW (1981) Gingival hyperplasia from dental braces. Cutis 28(2):149–150. [Crossref]
- Bishara SE , Barrett RD , Selim M (1993) Biodegradation of orthodontic appliances. Part II. Changes in the blood level of nickel. American Journal of Orthodontics and Dentofacial Orthopedics 103(2): 115–119. [Crossref]
- Eliades T , Trapalis C , Eliades G , Katsavrias E (2003) Salivary metal levels of orthodontic patients: a novel methodological and analytical approach. European Journal of Orthodontics 25(1): 103–106. [Crossref]
- Holmstrup P (1999) Non-plaque induced gingival lesions. Annals of Periodontology 4(1): 20–29. [Crossref]
- Limberger KM, Westphalen GH, Menezes LM, Medina-Silva R (2011) Cytotoxicity of orthodontic materials assessed by survival tests in Saccharomyces cerevisiae. Dental Materials 27(5): e81–e86. [Crossref]
- Shigeto N, Yanagihara T, Hamada T, Budtz-Jørgensen E (1989) Corrosion properties of soldered joints. Part I: electrochemical action of dental solder and dental nickel-chromium alloy. The Journal of Prosthetic Dentistry 62(5): 512–515. [Crossref]
- Mockers O, Deroze D, and Camps J (2002) Cytotoxicity of orthodontic bands, brackets and archwires in vitro. Dental Materials 18(4): 311–317. [Crossref]
- Berge M, Gjerdet NR, Erichsen ES (1982) Corrosion of silver soldered orthodontic wires. Acta Odontologica Scandinavica 40(2): 75–79. [Crossref]
- Fleischer M, Sarofim AF, Fassett DW, Hammond P, Shacklette HT, et al. (1974) Environmental impact of cadmium: a review by the panel on hazardous trace substances. Environmental Health Perspectives 7: 253–323. [Crossref]
- Novelli ELB, Hernandes TR, NovelliFilho JLVB, Barbosa LL (1998) Differential/combined effect of water contamination with cadmium and nickel on tissues of rats. Environmental Pollution 103(2-3): 295–300. [Crossref]
- Jin YH, Clark AB, Slebos RJC, Al-Refai H, Taylor JA, et al. (2003) Cadmium is a mutagen that acts by inhibiting mismatch repair. Nature Genetics 34(3): 326–329. [Crossref]
- Xie J, Shaikh ZA (2006) Cadmium induces cell cycle arrest in rat kidney epithelial cells in G2/M phase. Toxicology 224(1-2): 56–65. [Crossref]
- Huff J, Lunn RM, Waalkes MP, Tomatis L, Infante PF (2007) Cadmium-induced cancers in animals and in humans. International Journal of Occupational and Environmental Health 13(2): 202–212. [Crossref]
- Mikulewicz M, Chojnacka K (2011) Cytocompatibility of medical biomaterials containing nickel by osteoblasts: a systematic literature review. Biol. Trace Elem. Res 142: 865–889. [Crossref]
- Loe H, Theilade E, Jensen SB (1965) Experimental gingivitis in man. J Periodontol 36: 177-187. [Crossref]
- Ericsson I, Thilander B (1978) Orthodontic forces and recurrence of periodontal disease: An experimental study in the dog. Am J Orthod 74(1): 41-50. [Crossref]
- Ericsson I, Thilander B (1980) Orthodontic relapse in dentitions with reduced periodontal support: An experimental study in dogs. Eur JOrthod 2(1): 51-57. [Crossref]
- Ericsson I, Thilander B, Lindhe J, Okamoto H (1977) The effect of orthodontic tilting movements on the periodontal tissues of infected and non-infected dentitions in dogs. J ClinPeriodontol 4(4): 278-293. [Crossref]
- Polson AM, Subtelny JD, Meitner SW, Polson AP, Sommers EW, et al. (1988) Long-term periodontal status after orthodontic treatment. AmJ Orthod 93: 51-58. [Crossref]
- Amini F, Borzabadi Farahani A, Jafari A, Rabbani M (2008) In vivo study of metal content of oral mucosa cells in patients with and without fixed orthodontic appliances. Orthod Cranio fac Res 11(1):51-56. [Crossref]
- Kolokitha OE, Chatzistavrou E (2009) A severe reaction to Ni – containing orthodontic appliances. Angle Orthod 79(1): 186-192. [Crossref]
- Williams DF (2008) On the mechanisms of biocompatibility. Biomaterials 29(20): 2941–2953. [Crossref]
- Menezes LM, Quint˜ao CCA (2010) The release of ions from metallic orthodontic appliances. Seminars in Orthodontics 16(4): 282–292. [Crossref]
- Menezes LM, Campos LC, Quint˜ao CC, Bolognese AM (2004) Hypersensitivity to metals in orthodontics. American Journal of Orthodontics and Dentofacial Orthopedics126(1): 58–64. [Crossref]
- Andresen GF, Morrow RE (1978) Laboratory and clinical analyses of nitinol wire. Am J Orthod 73(2): 142–151. [Crossref]
- Schuster G, Reichle R, Bauer RR, Schopf PM (2004) Allergies induced by orthodontic alloys: incidence and impact on treatment. Results of a survey in private orthodontic offices in the Federal State of Hesse, Germany. J Orofac Orthop 65(1): 48–59. [Crossref]
- Eliades T (2007) Orthodontic materials research and applications: Part 2: current status and projected future developments in materials and biocompatibility. Am J Orthod Dentofacial Orthop 131(2): 253–262. [Crossref]
- Pereira BR, Tanaka OM, Lima AAS, Guariza-Filho O, Maruo H, et al. (2009) Metal and ceramic bracket effects on human buccal mucosa epithelial cells. Angle Orthod 79(2): 373–379. [Crossref]
- Malkoc S, Corekci B, Ulker HE, Yalçin M, Sengün A (2010) Cytotoxic effects of orthodontic composites. Angle Orthod 80(4): 759–764. [Crossref]
- Toy E, Malkoc S, Corekci B, Bozkurt BS, Hakki SS (2014) Real-time cell analysis of the cytotoxicity of orthodontic brackets on gingival fibroblasts. J Appl Biomater Funct Mater 12(3): 248 -255. [Crossref]







