Niels Bohr developed the model of the atom in which energy levels of electrons are discrete, that electrons revolve in stable orbits around the atomic nucleus and can quantum jump from the second highest energy level (orbital) to the highest energy level (outermost orbital). As an electron drops back from the outermost orbital, the energy it loses is emitted as a photon. The 6 elements in the periodic table in which quantum jumps cannot occur are colorless. All 90 elements in which quantum jumps occur have an intrinsic color that is imparted to them by the predetermined steady frequency of each quantum jump. Thus, quantum jumping is the only factor which explains the specific color of every element in the periodic table.
Atomic Physics; Photons; Quantum Mechanics; Quantum Jumping
The photon is a minute energy packet. of electromagnetic radiation. This concept originated in Albert Einstein’s explanation of the photoelectric effect, in which he proposed the existence of discrete energy packets during the transmission of light [1]. The energy of a photon depends on radiation frequency. There are photons of all energies from high energy gamma- and X-rays, through visible light, to low-energy infrared and radio waves. All photons travel at the speed of light [2].
A photon is massless, stable, has no electric charge, and is considered to be an elementary particle [3]. If the photon were not entirely massless, it would be unable to travel at the speed of light.
Niels Bohr developed the model of the atom in which energy levels of electrons are discrete, that electrons revolve in stable orbits around the atomic nucleus and can quantum jump from the second highest energy level (orbital) to the highest energy level (outermost orbital). As an electron drops back from the outermost orbital, the energy it loses is emitted as a photon [4].
Unlike an electromagnetic wave, a photon cannot be of a color. Instead, a photon corresponds to light of a given color depending on its energy of emission. The human eye perceives red light at approximately 2 electric volts (eV), blue light at about 3 eV, with all visible colors in between.
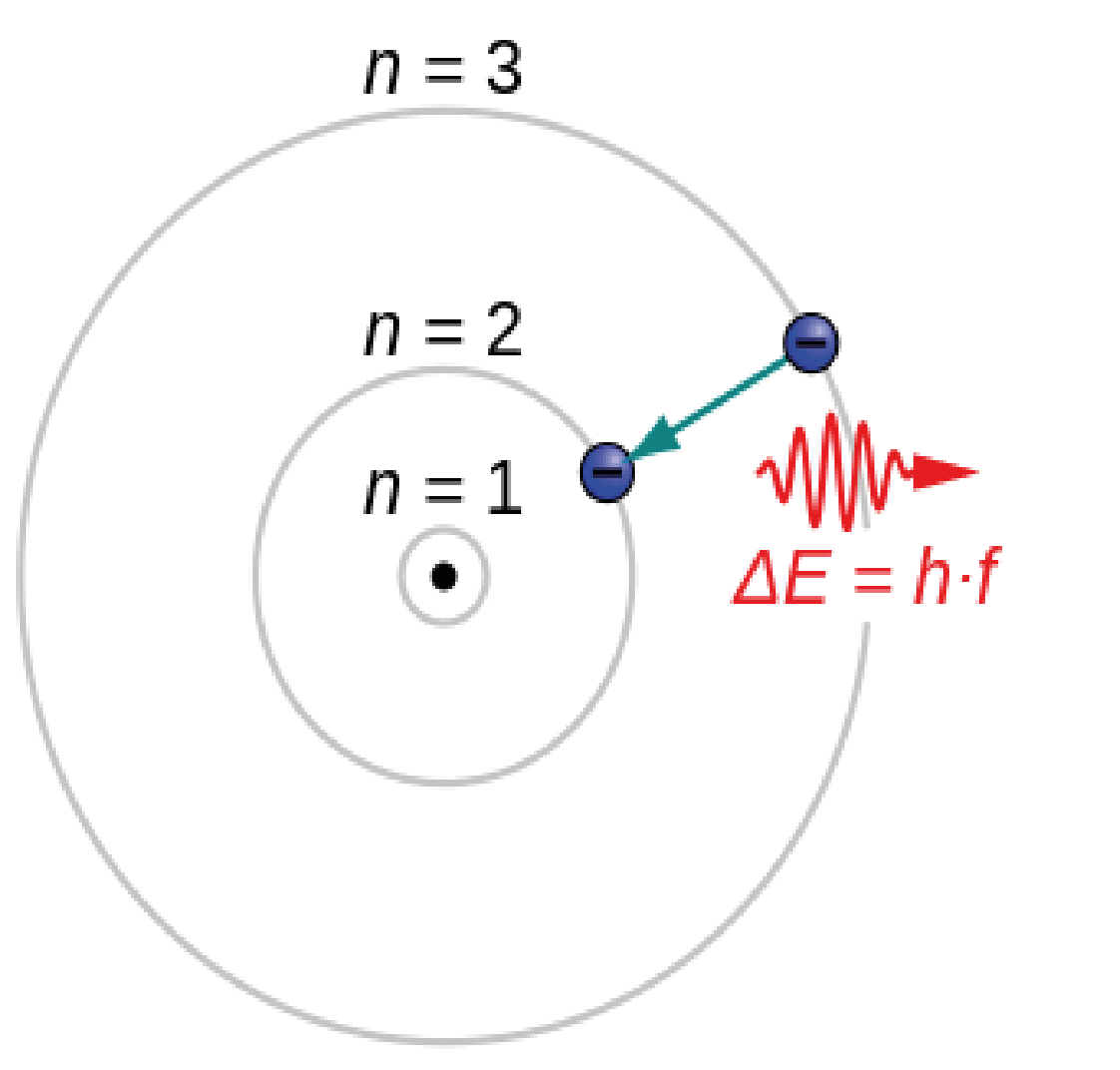
Atomic Structure
Figure 1: An electron moving from quantum level n=3 to n = 2 and releasing a photon.
Every element in the periodic table has a unique atomic structure that is determined by its electron configuration [5], Electron configurations describe each electron as moving independently in an orbital, in an average field created by all other orbitals [6]. Electrons are able to move from one configuration to another by means of quantum jumping. Typically, it takes a few nanoseconds or less for an electron to jump from its quantized energy level to the next highest quantized level [7].
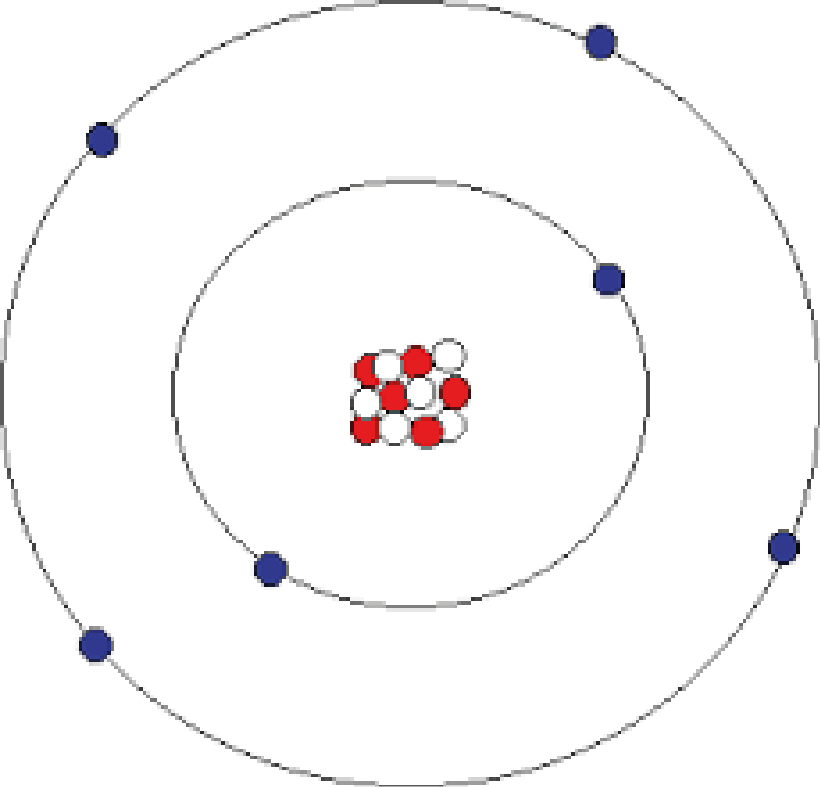
Figure 2. Structure of the Carbon Atom
In the carbon atom there are two electrons in the 1st level orbital and four in the 2nd level orbital. At what may be millions of times per second, an electron from the first level jumps to the second level and jumps back again. As it returns to its former level, it emits a photon.
Quantum jumps are always to the highest energy level orbital, and always return to the second highest level orbital from whence they came. Quantum jumps occur only in atoms in which outer orbitals have space to accommodate an additional electron.
Quantum jumping cannot happen in hydrogen and helium because these elements have only one orbital. Hydrogen and helium do not emit photons. Thus, they are invisible to us.
Quantum jumping also cannot happen in elements which have full outer orbitals. These include neon, argon, krypton, xenon, and radon. None of these gases emits photons. All of them are invisible to us.
Quantum jumps, long assumed to be instantaneous and random, have recently been discovered to be gradual and predictable [8]. With a super high-speed monitoring system, researchers can spot when a quantum jump is about to take place. Their experimental results indicate that the jump evolution is continuous, coherent, and deterministic [9].
Elements with Only One Orbital
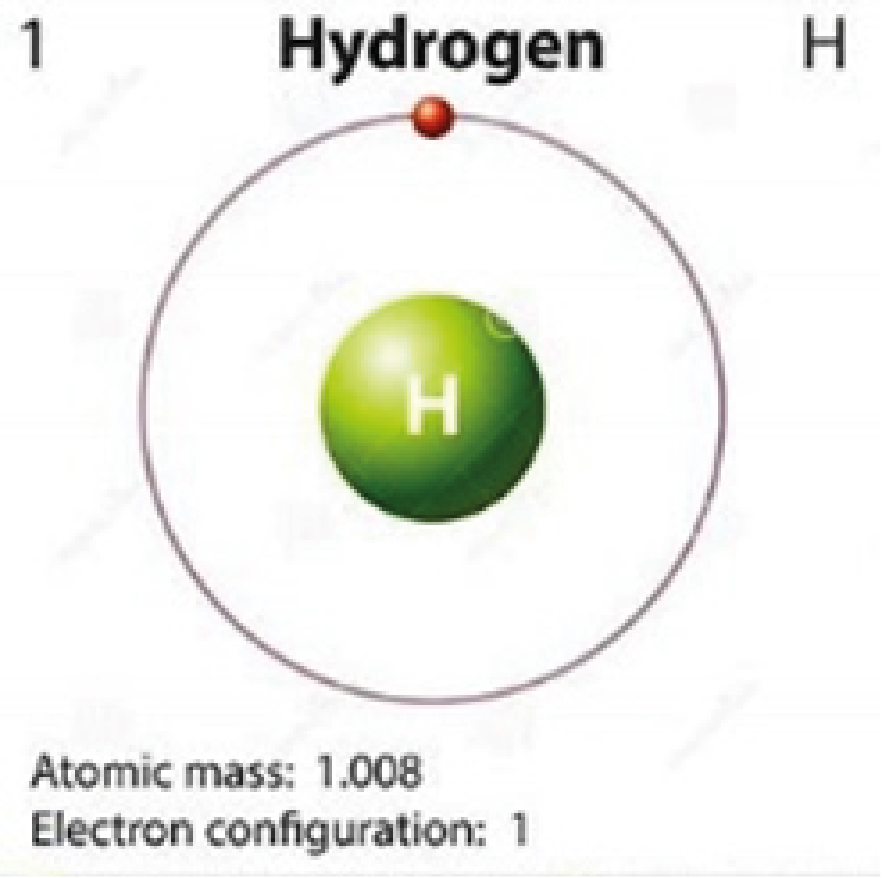
Hydrogen H |
Colorless |
 |
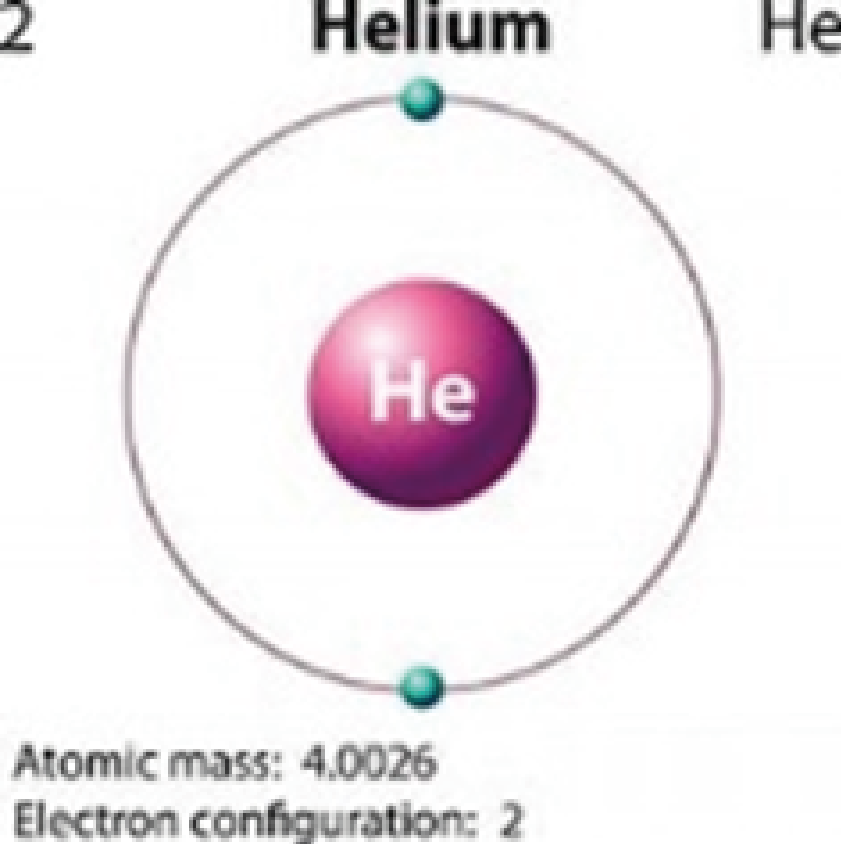
Helium He |
Colorless |
|
Because hydrogen and helium have only one orbital, quantum jumping is impossible for them. This is why these elements are colorless.
Elements with Complete Outer Orbitals
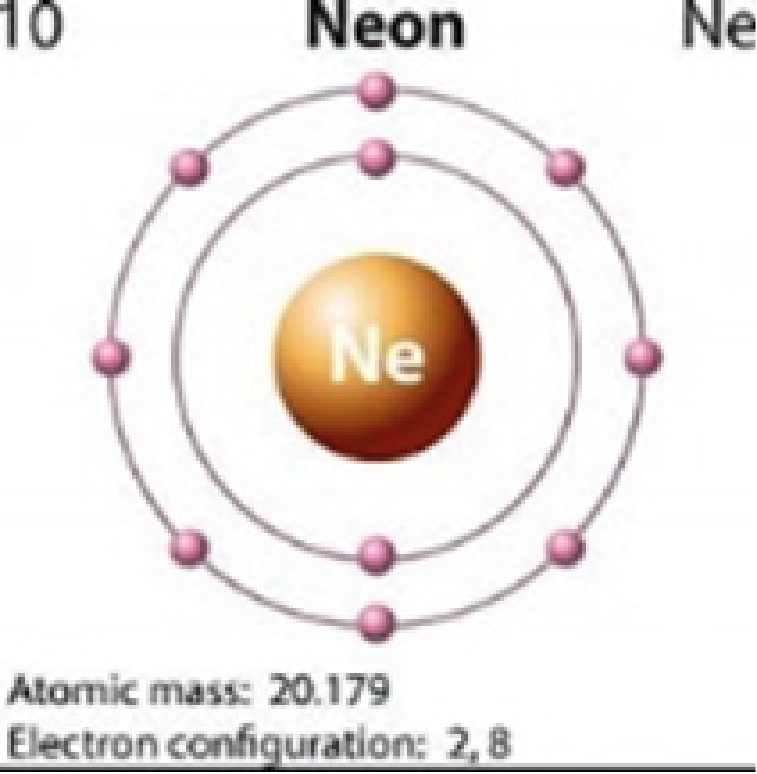
Neon
2,8 |
Colorless |
 |
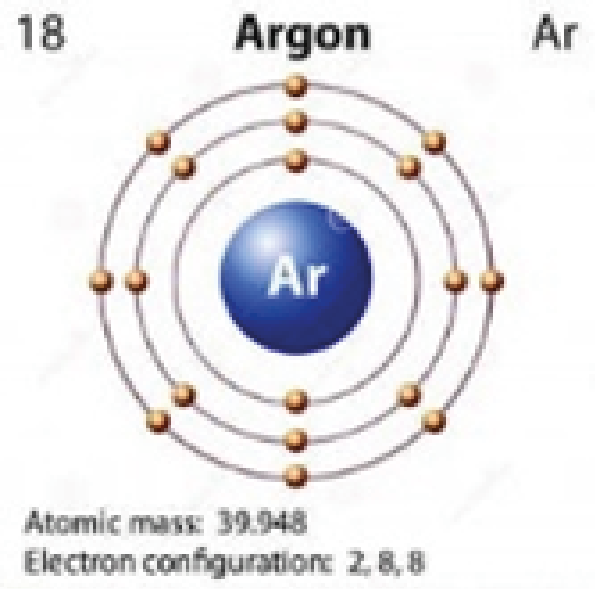
Argon
2,8,8 |
Colorless |
 |
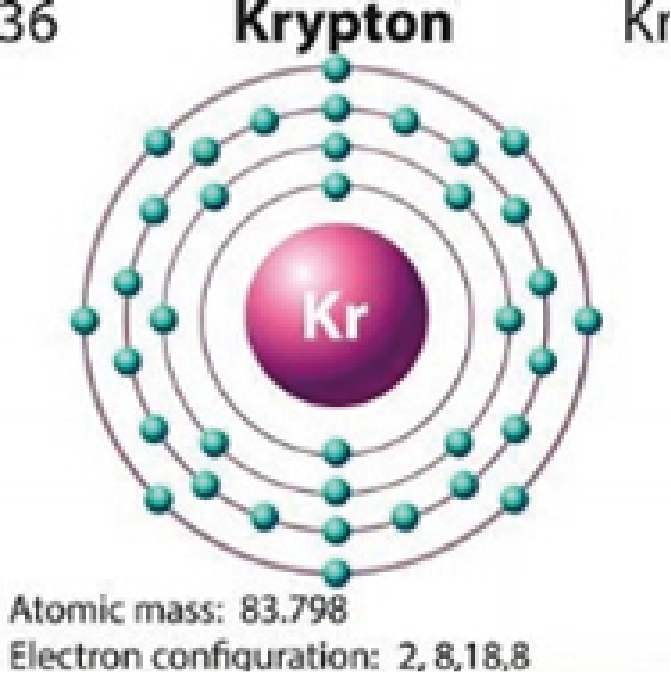
Krypton
2,8,18,8 |
Colorless |
 |
Xenon
2,8,18,18,8
|
Colorless |
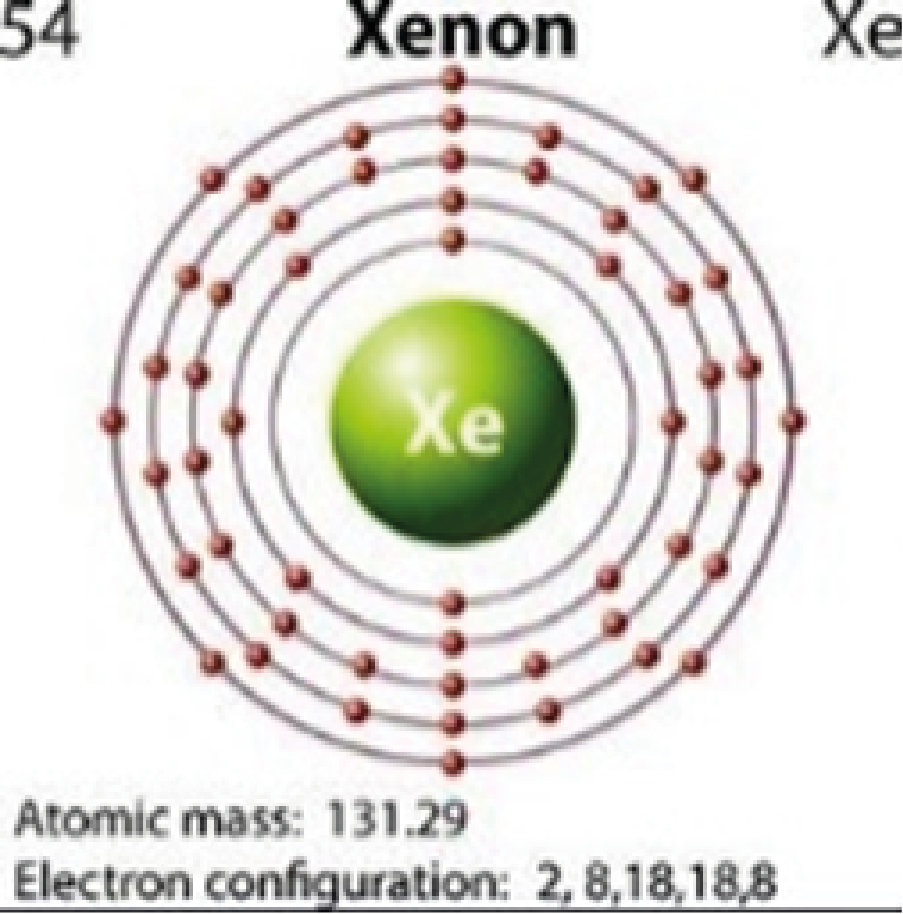 |
Quantum jumping is also impossible for elements that that have full outer orbitals (i.e., neon, argon, krypton, xenon). Their outer orbitals cannot accommodate the addition of another electron. This is why these elements are colorless.
How the Eye Perceives Colors
Color is the perception of the energy and frequencies of light that reach our eyes. Light receptors within the eye detect light over a range of frequencies and transmit messages to the brain, which interprets each frequency as a specific color.
Table 1: The Visible Spectrum
Color |
Frequency [10] |
Energy [10] |
Red (limit) |
4.29 x 1014 Hz |
1.77 eV |
Red |
4.62 x 1014 Hz |
1.91 eV |
Orange |
5.00 x 1014 Hz |
2.06 eV |
Yellow |
5.16 x 1014 Hz |
2.14 eV |
Green |
5.45 x 1014 Hz |
2.25 eV |
Cyan |
5.99 x 1014 Hz |
2.48 eV |
Blue |
6.66 x 1014 Hz |
2.75 eV |
Violet (limit) |
7.50 x 1014 Hz |
3.10 eV |
Table 2: Colors of the Elements
|
Electrons |
Element |
|
Configuration |
Color |
3 |
Lithium |
Li |
2,1 |
silvery white |
4 |
Beryllium |
Be |
2,2 |
white-grey metallic |
5 |
Boron |
B |
2,3 |
black-brown |
6 |
Carbon |
C |
2,4 |
black metallic |
7 |
Nitrogen |
N |
2,5 |
bluish green (as a solid) |
8 |
Oxygen |
O |
2,6 |
pale blue (as a solid) |
9 |
Fluorine |
F |
2,7 |
very pale yellow |
11 |
Sodium |
Na |
2,8,1 |
silvery white metallic |
12 |
Magnesium |
Mg |
2,8,2 |
shiny grey |
13 |
Aluminum |
Al |
2,8,3 |
silvery grey metallic |
14 |
Silicon |
Si |
2,8,4 |
blue-grey |
15 |
Phosphorus |
P |
2,8,5 |
white, red, violet, or black metallic |
16 |
Sulphur |
S |
2,8,6 |
lemon yellow |
17 |
Chlorine |
Cl |
2,8,7 |
pale yellow-green |
19 |
Potassium |
K |
2,8,8,1 |
silvery white |
20 |
Calcium |
Ca |
2,8,8,2 |
dull grey-silver with pale yellow tint |
21 |
Scandium |
Sc |
2,8,8,3 |
silvery white |
22 |
Titanium |
Ti |
2,8,8,4 |
silvery grey-white metallic |
23 |
Vanadium |
V |
2,8,8,5 |
blue-silver-grey |
24 |
Chromium |
Cr |
2,8,8,6 |
silvery metallic |
25 |
Manganese |
Mn |
2,8,8,7 |
silvery metallic |
26 |
Iron |
Fe |
2,8,8,8 |
lustrous metallic with greyish tinge |
27 |
Cobalt |
Co |
2,8,8,9 |
lustrous bluish grey |
28 |
Nickel |
Ni |
2,8,8,10 |
lustrous metallic silver with gold tinge |
29 |
Copper |
Cu |
2,8,8,11 |
red-orange metallic luster |
30 |
Zinc |
Zn |
2,8,8,12 |
silver-grey |
31 |
Gallium |
Ga |
2,8,8,13 |
silvery blue |
32 |
Germanium |
Ge |
2,8,8,14 |
greyish white |
33 |
Arsenic |
As |
2,8,8,15 |
grey |
34 |
Selenium |
Se |
2,8,8,16 |
grey metallic |
35 |
Bromine |
Br |
2,8,8,17 |
reddish-brown |
37 |
Rubidium |
Rb |
2,8,8,18,1 |
whitish grey |
38 |
Strontium |
Sr |
2,8,8,18,2 |
silvery white metallic, pale yellow tint |
39 |
Yttrium |
Y |
2,8,8,18,3 |
silvery white |
40 |
Zirconium |
Zr |
2,8,8,18,4 |
silvery white |
41 |
Niobium |
Nb |
2,8,8,18,5 |
grey metallic |
42 |
Molybdenum |
Mo |
2,8,8,18,6 |
grey metallic |
43 |
Technetium |
Te |
2,8,8,18,7 |
shiny grey |
44 |
Ruthenium |
Ru |
2,8,8,18,8 |
silvery white metallic |
45 |
Rhodium |
Rh |
2,8,8,18,9 |
silvery white metallic |
46 |
Palladium |
Pd |
2,8,8,18,10 |
silvery white |
47 |
Silver |
Ag |
2,8,8,18,11 |
silver |
48 |
Cadmium |
Cd |
2,8,8,18,12 |
silvery bluish grey metallic |
49 |
Indium |
In |
2,8,8,18,13 |
silvery grey, lustrous |
50 |
Tin |
Sn |
2,8,8,18,14 |
silvery white |
51 |
Antimony |
Sb |
2,8,8,18,15 |
silvery grey, lustrous |
52 |
Tellurium |
Te |
2,8,8,18,16 |
silvery grey, lustrous |
53 |
Iodine |
I |
2,8,8,18,17 |
metallic grey, lustrous (as a solid) |
55 |
Cesium |
Cs |
2,8,8,18,18,1 |
silvery golden |
56 |
Barium |
Ba |
2,8,8,18,18,2 |
silvery grey, pale yellow tint |
57 |
Lanthanum |
La |
2,8,8,18,18,3 |
silvery white |
58 |
Cerium |
Ce |
2,8,8,18,18,4 |
silvery white |
59 |
Praseodymium |
Pr |
2,8,8,18,18,5 |
greyish white |
60 |
Neodymium |
Nd |
2,8,8,18,18,6 |
silvery white |
61 |
Promethium |
Pm |
2,8,8,18,18,7 |
silvery white metallic |
62 |
Samarium |
Sm |
2,8,8,18,18,8 |
silvery white |
63 |
Europium |
Eu |
2,8,8,18,18,9 |
silvery white, pale yellow tint |
64 |
Gadolinium |
Gd |
2,8,8,18,18,10 |
silvery white |
65 |
Terbium |
Tb |
2,8,8,18,18,11 |
silvery white |
66 |
Dysprosium |
Dy |
2,8,8,18,18,12 |
silvery white |
67 |
Holmium |
Ho |
2,8,8,18,18,13 |
silvery white |
68 |
Erbium |
Er |
2,8,8,18,18,14 |
silvery white |
69 |
Thulium |
Tm |
2-8-8-18-18-15 |
silvery grey |
70 |
Ytterbium |
Yb |
2,8,8,18,18,16 |
silvery white, pale yellow tint |
71 |
Lutetium |
Lu |
2,8,8,18,18,17 |
silvery white |
72 |
Hafnium |
Hf |
2,8,18,32,10,2 |
steel grey |
73 |
Tantalum |
Ta |
2,8,18,32,10,3 |
grey-blue |
74 |
Tungsten |
W |
2,8,18,32,10,4 |
greyish white, lustrous |
75 |
Rhenium |
Re |
2,8,18,32,10,5 |
silvery-greyish |
76 |
Osmium |
Os |
2,8,18,32,10,6 |
silvery, blue cast |
77 |
Iridium |
Ir |
2,8,18,32,10,7 |
silvery white |
78 |
Platinum |
Pt |
2,8,18,32,17,1 |
silvery white |
79 |
Gold |
Au |
2,8,18,32,18,1 |
metallic yellow |
80 |
Mercury |
Hg |
2,8,18,32,18,2 |
silvery liquid, shiny |
81 |
Thallium |
Ti |
2,8,18,32,18,3 |
silvery white |
82 |
Lead |
Pb |
2,8,18,32,18,4 |
metallic grey |
83 |
Bismuth |
Bi |
2,8,18,32,18,5 |
brownish silver, lustrous |
84 |
Polonium |
Po |
2,8,18,32,18,6 |
silvery grey |
85 |
Astatine |
At |
2,8,18,32,18,7 |
silvery |
87 |
Francium |
Fr |
2,8,18,32,18,8,1 |
silvery |
88 |
Radium |
Ra |
2,8,18,32,18,8,2 |
silvery white metallic |
89 |
Actinium |
Ac |
2,8,18,32,18,9,2 |
silvery white, blue glow |
90 |
Thorium |
Th |
2,8,18,32,18,10,2 |
silvery |
91 |
Protactinium |
Pa |
2,8,18,32,20,9,2 |
silvery metallic luster |
92 |
Uranium |
U |
2,8,18,32,21,9,2 |
silvery grey metallic |
93 |
Neptunium |
Np |
2,8,18,32,22,9,2 |
silvery white |
94 |
Plutonium |
Pu |
2,8,18,32,24,8,2 |
silvery white |
95 |
Americium |
Am |
2,8,18,32,25,8,2 |
silvery white |
96 |
Curium |
Cm |
2,8,18,32,25,9,2 |
silvery metallic |
Five of the seven elements with one electron in their outer orbital are silvery white in color (Li, Na, K, Pt, Fr). One is silvery golden (Cs). One is whitish grey (Rb).
Five of the eight elements with two electrons in their outer orbital have grey in their color (Be, Mg, Ca, Ba, Hf). Three are of a silvery color (Sr, Hg, Ra).
All elements having seven orbitals are silvery in color (Fr, Ra, Ac, Th, Pa, U, Np, Pu, Am, Cm).
Each element in the periodic table has a steady frequency with which its electrons jump back and forth between orbitals giving off light. The frequency of each quantum jump generates a unique frequency for that element, which frequency is experienced by the human eye as a specific color.
The 6 elements in the periodic table in which quantum jumps cannot occur are colorless. All 90 elements in which quantum jumps do occur have an intrinsic color that is imparted to them by the predetermined steady frequency of each quantum jump. Thus, quantum jumping is the only factor which explains the specific color of every element in the periodic table. Light receptors within the eye detect the frequencies of photons emitted by the elements and transmit messages to the brain, which interprets each frequency as a given color.
- Einstein A (1905) On a heuristic point of view concerning the generation and transformation of light. Annals of Physics 17: 132-148.
- Augustyn A (2022) Photon. Encyclopedia Britannica.
- Frisch DH., Thorndike AM (1964) Elementary Particles. Princeton NJ: David Van Nostrand 22.
- Itano WM., Bergquist JC., Wineland DJ (2015) Early observations of macroscopic quantum jumps in single atoms. International Journal of Mass Spectrometry 377: 403.
- Langmuir I (1919) The arrangement of electrons in atoms and molecules. Journal of the American Chemical Society 41: 868-934.
- Stoner EC (1924) The distribution of electrons among atomic levels. Philosophical Magazine 48: 719-736.
- Vijay R., Slichter DH., Siddiqi I (2011) Observations of quantum jumps in a superconducting artificial atom. Physical Review Letters 106: 110502.
- Ball P (2019) Quantum leaps, long assumed to be instantaneous, take time. Quanta Magazine.
- Minev ZK., Mundhada SO (2019) To catch and reverse a quantum jump mid-flight. Nature 570: 200-204.
- Editors of Encyclopedia. The visible spectrum. Brittanica.com.








